Systematic Approaches on Extractable and Leachable Study Designs in Pharmaceuticals and Medical Devices: A Review | Journal of Packaging Technology and Research

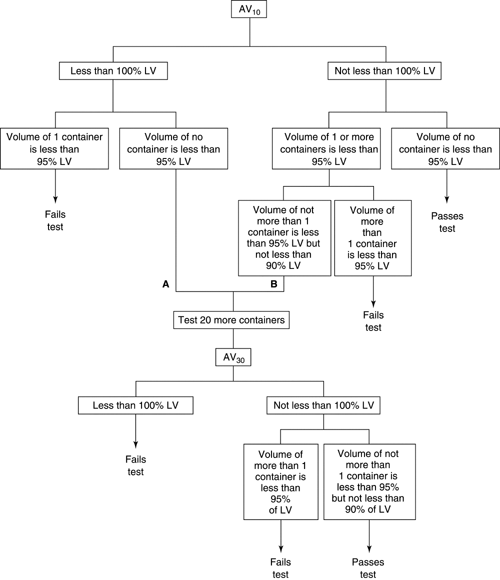
5.6 EXTRACTABLE VOLUME FOR PARENTERAL PREPARATIONS … / 5-6-extractable- volume-for-parenteral-preparations.pdf / PDF4PRO
Update on USP's General Chapters on Biological Reactivity, Extractables and Leachables, and Glass Containers

Non-volatile extractable analysis of prefilled syringes for parenteral administration of drug products - ScienceDirect