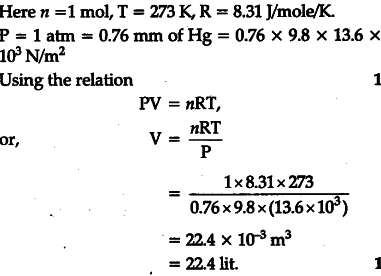
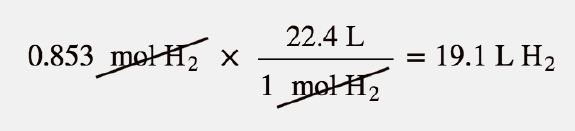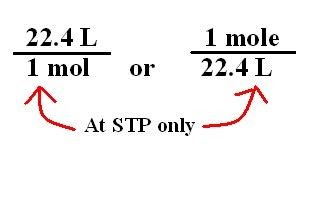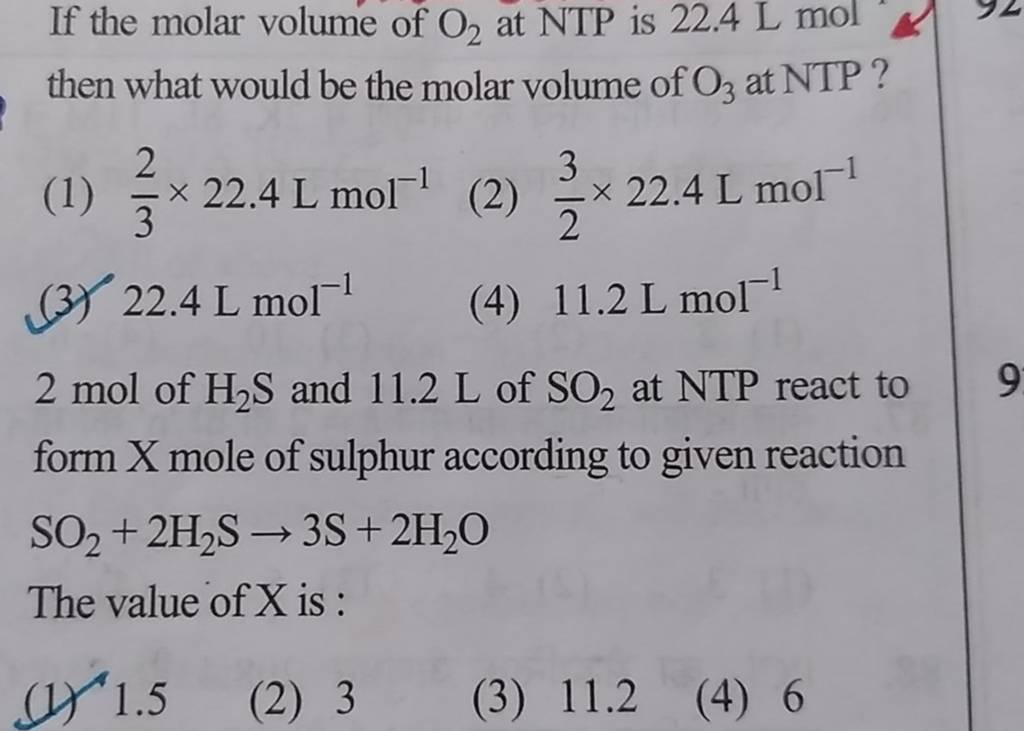Q. 2.17: One mole of an ideal gas at standard temperature and pressure occupies 22.4 L(molar volume) - YouTube

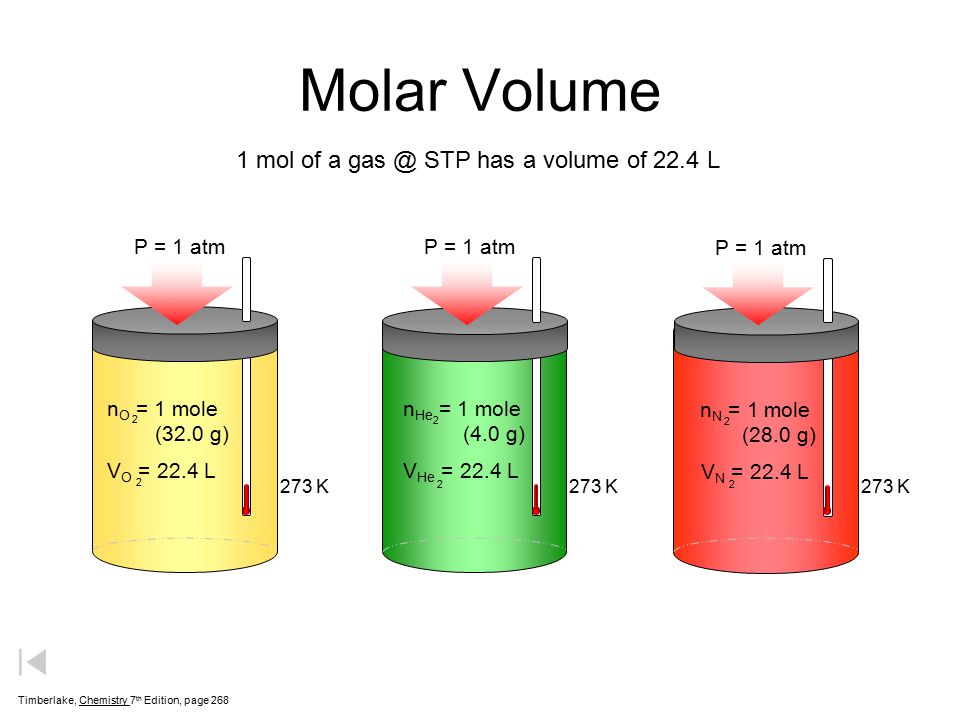
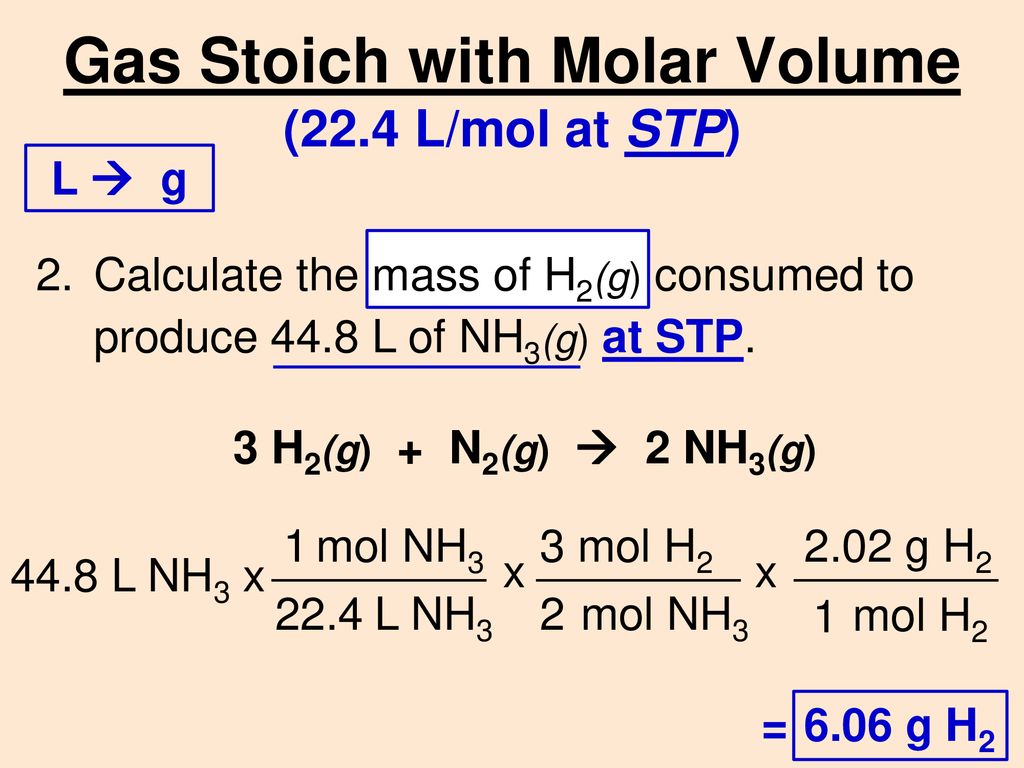
Gases & Stoichiometry. Molar Volume 1 mol of gas = 22.4 L molar volume What volume would be occupied by 0.77 moles of helium gas at STP? - ppt download

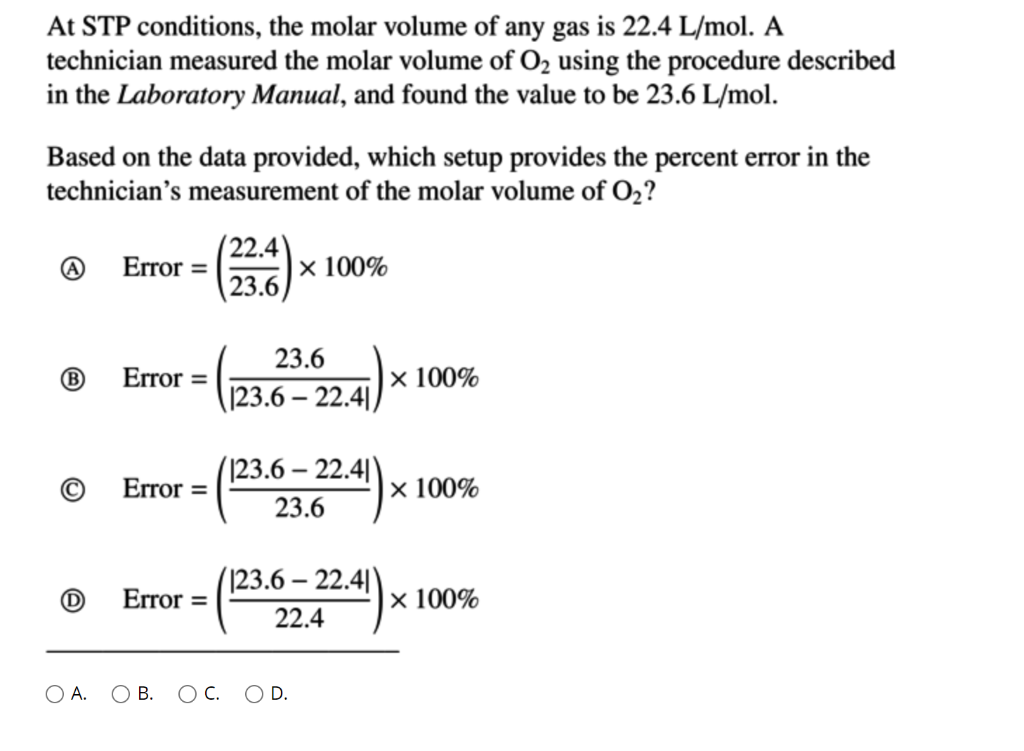
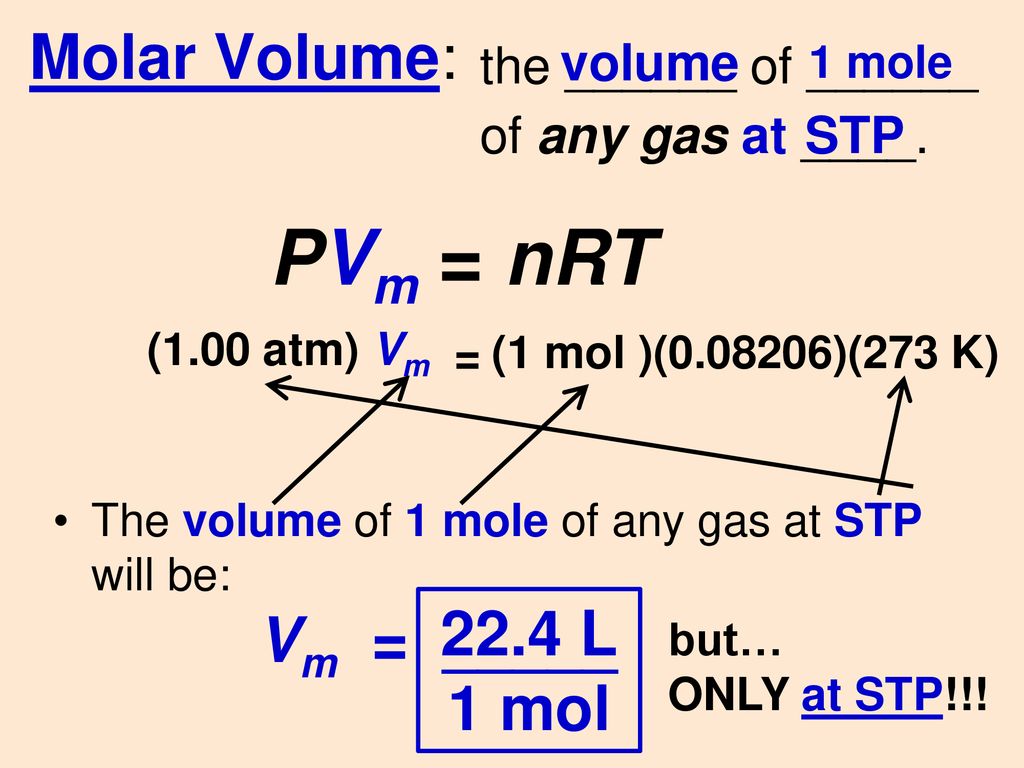
SOLVED: Using the ideal gas law, show that at STP, the molar volume of an ideal gas is 22.4 L. | Numerade

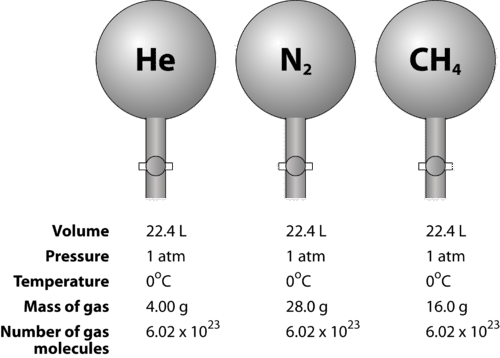
SOLVED: Avogadro's Law and Molar Volume at STP = 22.4 L (1 mole of any gas occupies 22.4 liters at STP). (40 L) 50 g of nitrogen (N2) has a volume of 2.8 L.