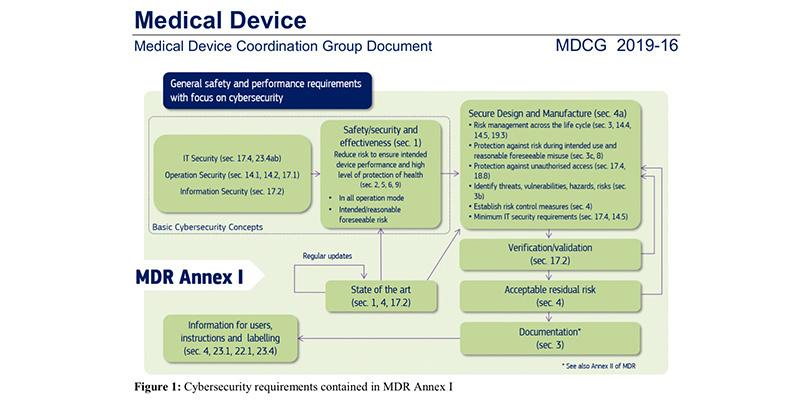
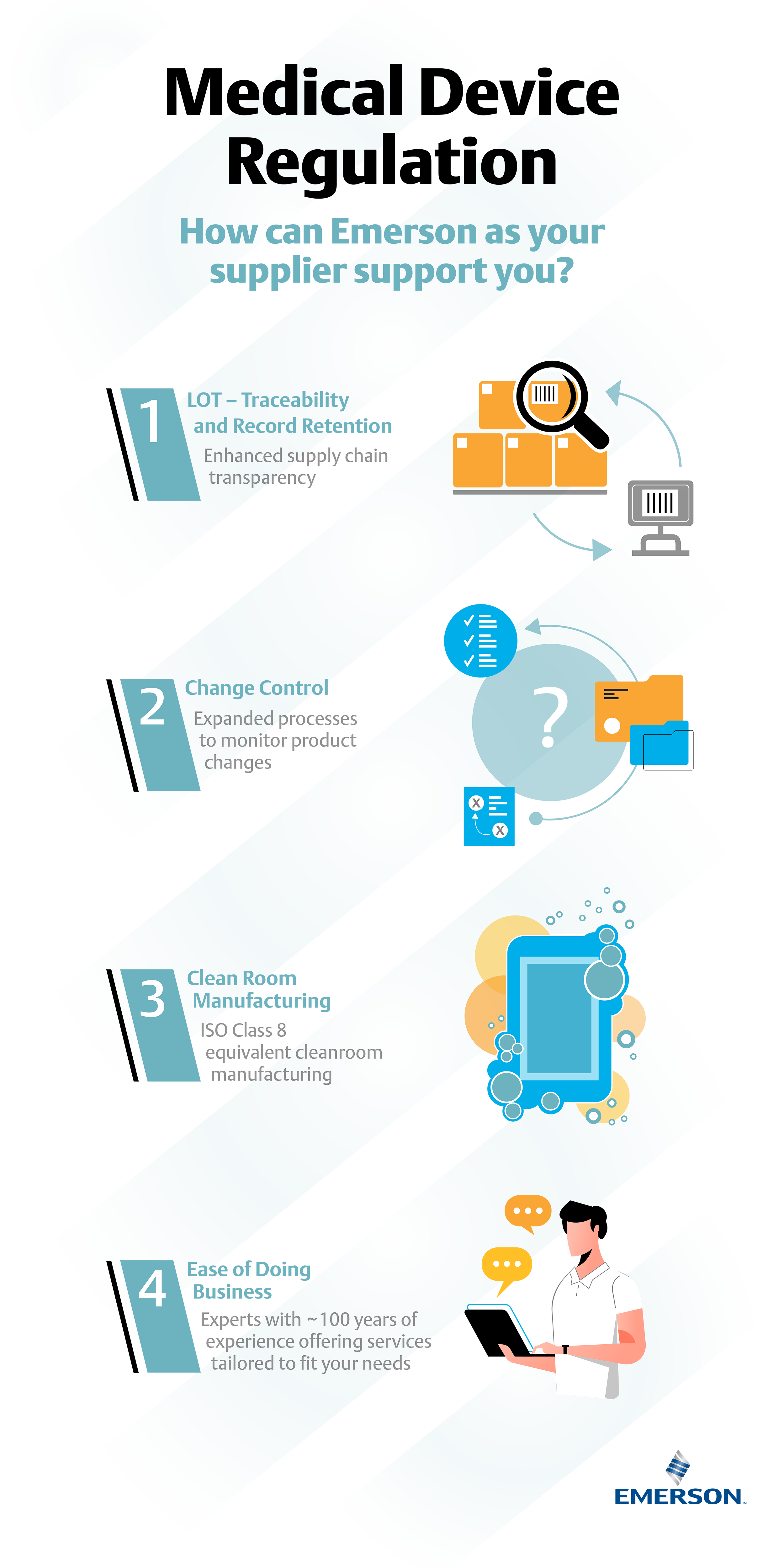
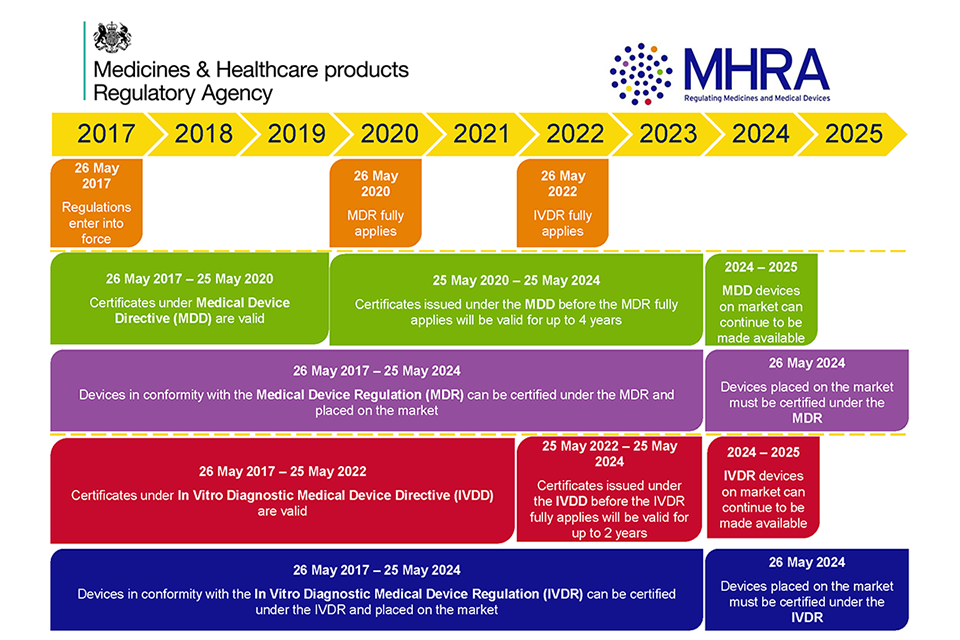
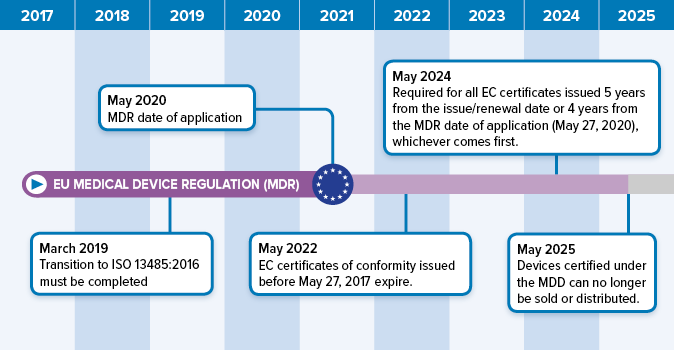
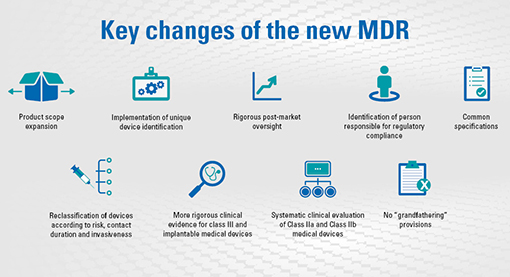
DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products

MDR Medical Devices Regulation 745/2017: Regolamento Europeo per i dispositivi medici in vigore da Maggio 2020 - EMD112 - Prodotti e Formazione Salvavita