Manual on borderline and classification for medical devices under Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnostic medical devices - Formiventos

PDF) BORDERLINE AND CLASSIFICATION IN THE COMMUNITY REGULATORY FRAMEWORK FOR MEDICAL DEVICES – BRIEF REVIEW ON SOME DENTISTRY PRODUCTS | Maya Lyapina - Academia.edu

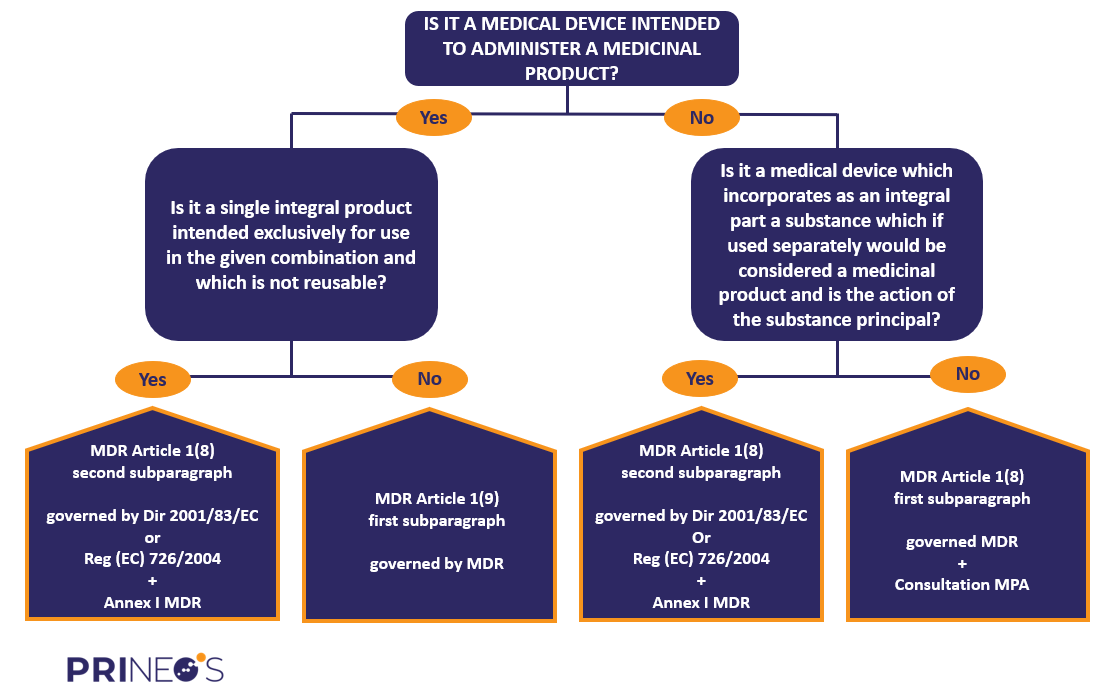
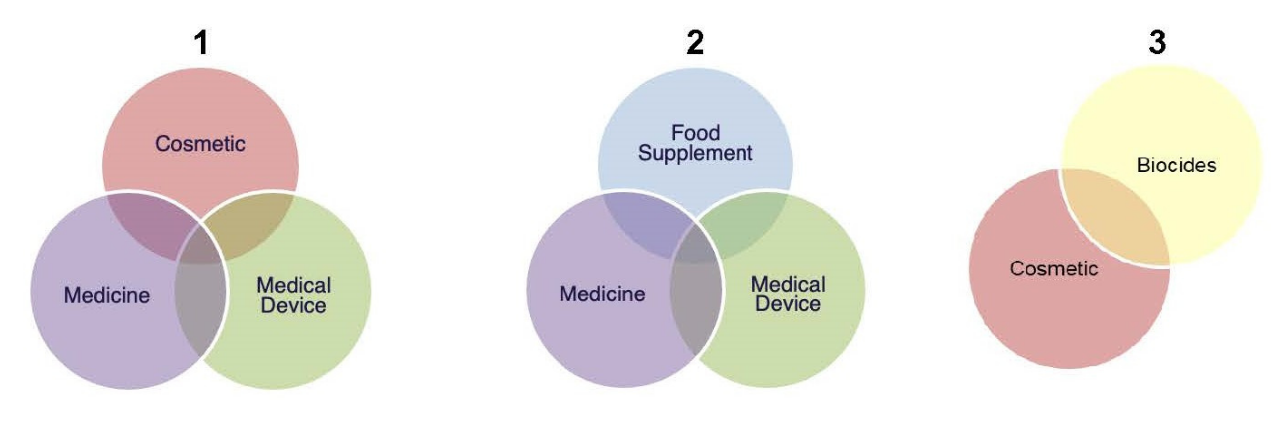
EU Medical Device Coordination Group (MDCG) approves “Guidance” on the borderline between medical devices and medicinal products in order to support the uniform application of Regulation (EU) 2017/745 (MDR) across the European