a Overview of the acidity and basicity of metal oxides. b Possible CO2... | Download Scientific Diagram
A metal oxide has the formula M2O it can be thermally decomposed to the metal and oxygen 5.8 g of the metal oxide forms 400mh of oxygen gas on complete decomposition .the
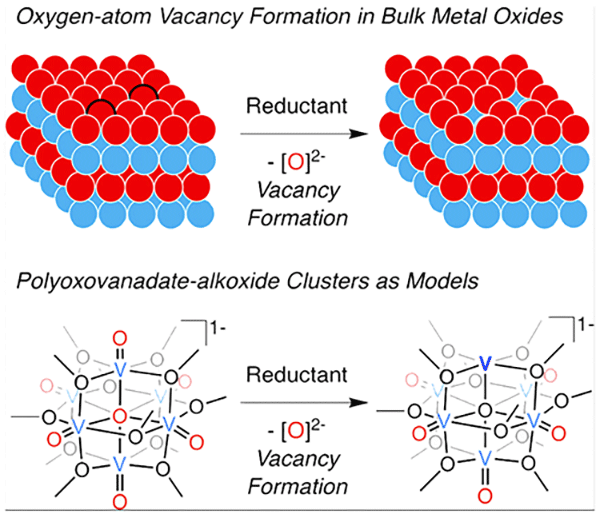
Matson Group 'cleaves' oxygen from surface of metal oxide, enhancing reactivity : News & Events : Department of Chemistry : University of Rochester
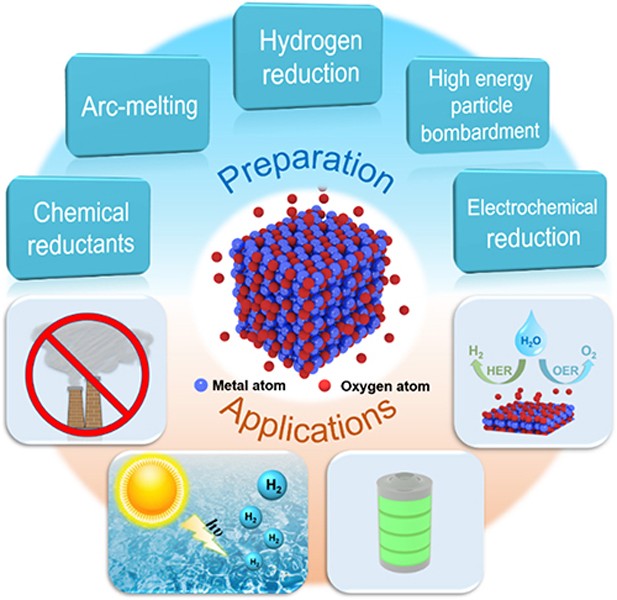
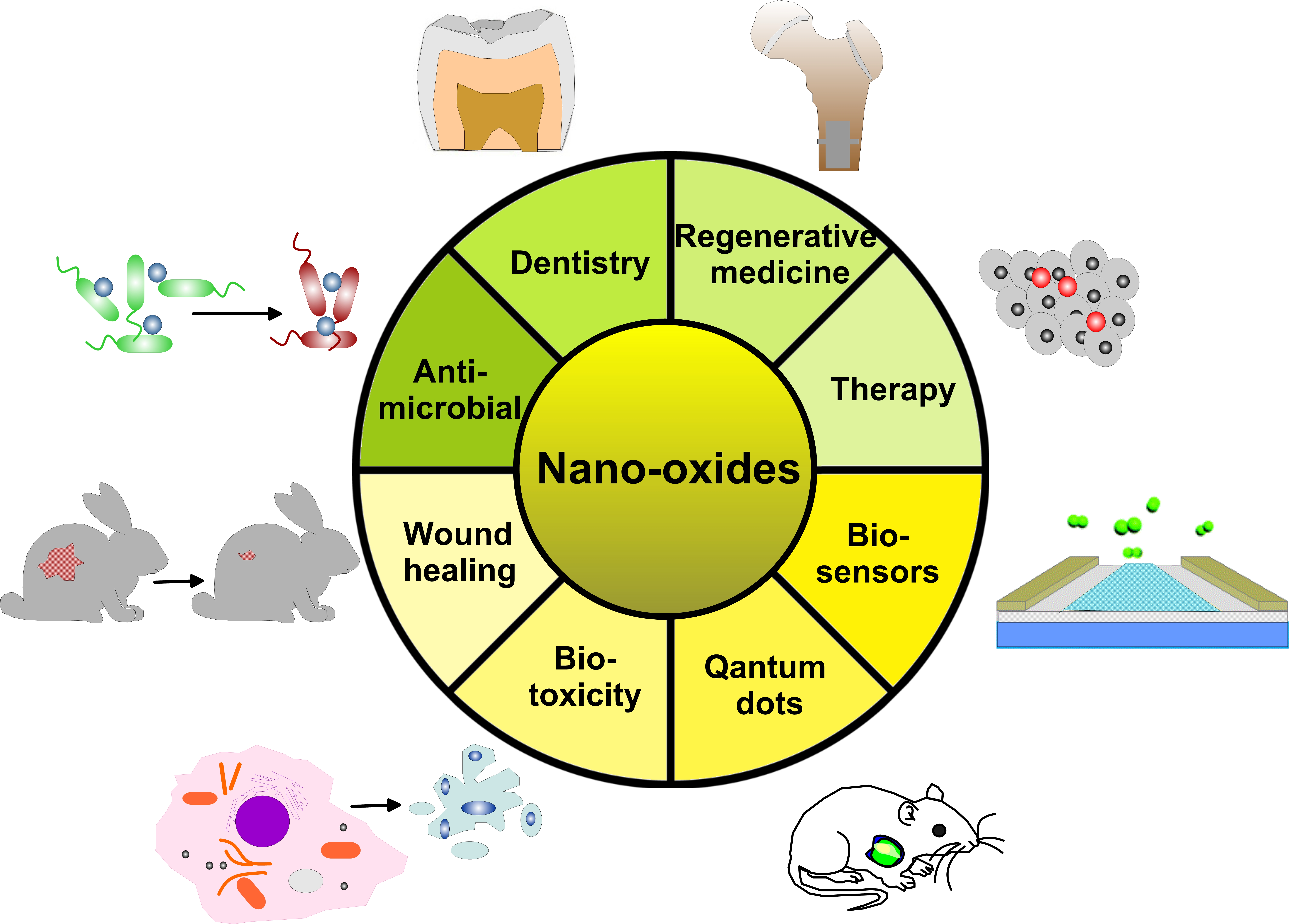
Oxygen-deficient metal oxides: Synthesis routes and applications in energy and environment | Nano Research

Difference Between Metal Oxides and Non Metal Oxides | Definition, Properties, Different Types, Differences

Two metallic oxides contain 27.6% and 30% oxygen respectively. If the formula of the first oxide is X3O4(X is any metal) , then the formula of the second oxide will be ? - 4jyhffss







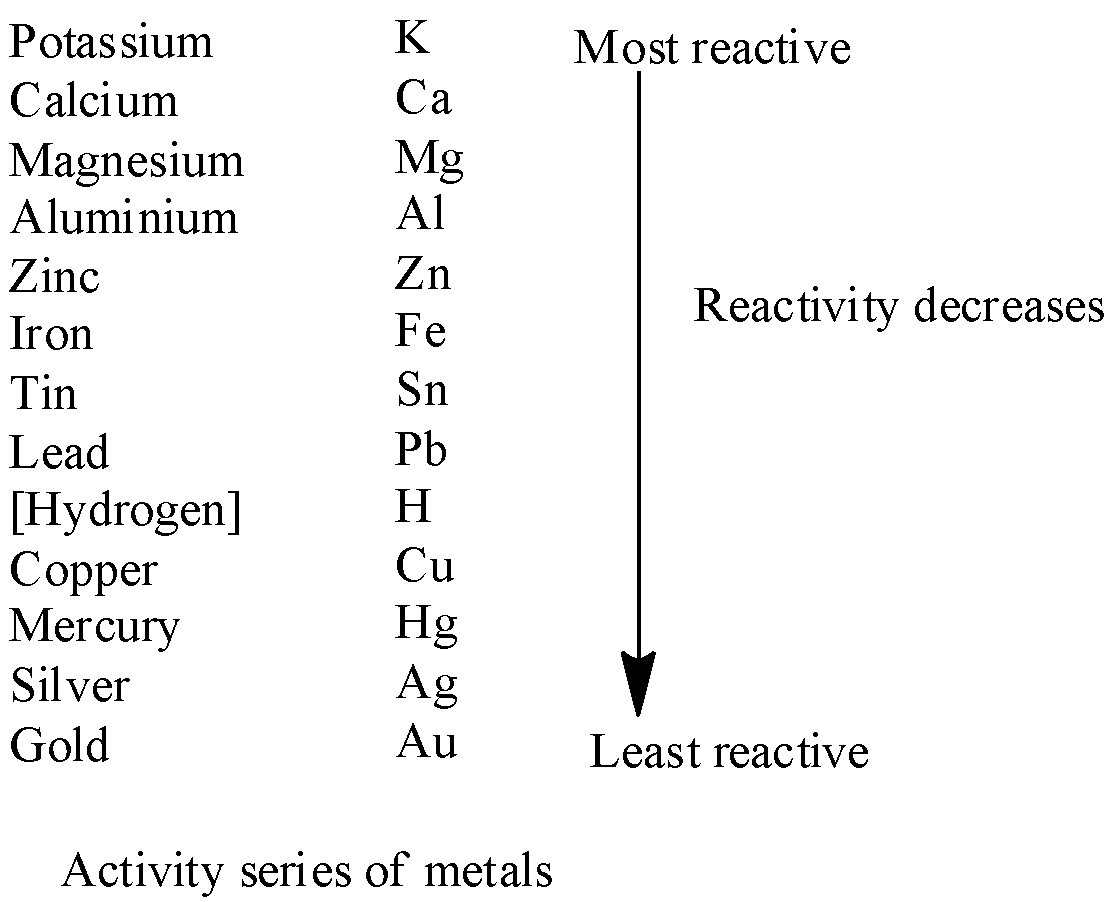
![Chemical Properties of Metals [with Reaction Examples] - Teachoo Chemical Properties of Metals [with Reaction Examples] - Teachoo](https://d1avenlh0i1xmr.cloudfront.net/96497642-1b9f-4e2d-b9dd-61e9e45812cf/metal-oxides---teachoo.png)




![MCQ] Which of the statements is not correct? All metal oxides react MCQ] Which of the statements is not correct? All metal oxides react](https://d1avenlh0i1xmr.cloudfront.net/cbf9e2bf-7faa-4285-8706-554b06bb04d1/reaction-of-metals-with-acids---teachoo.jpg)