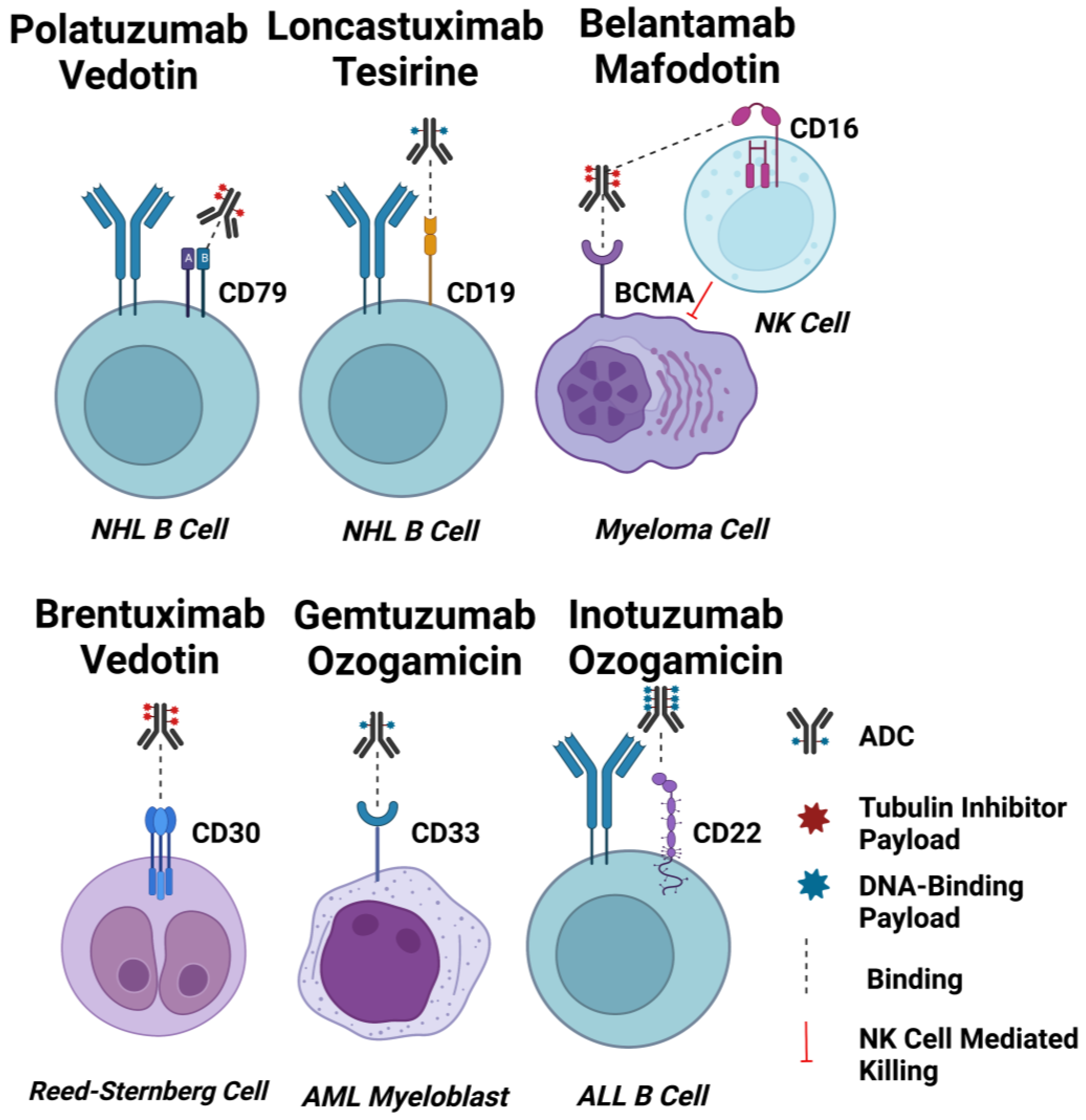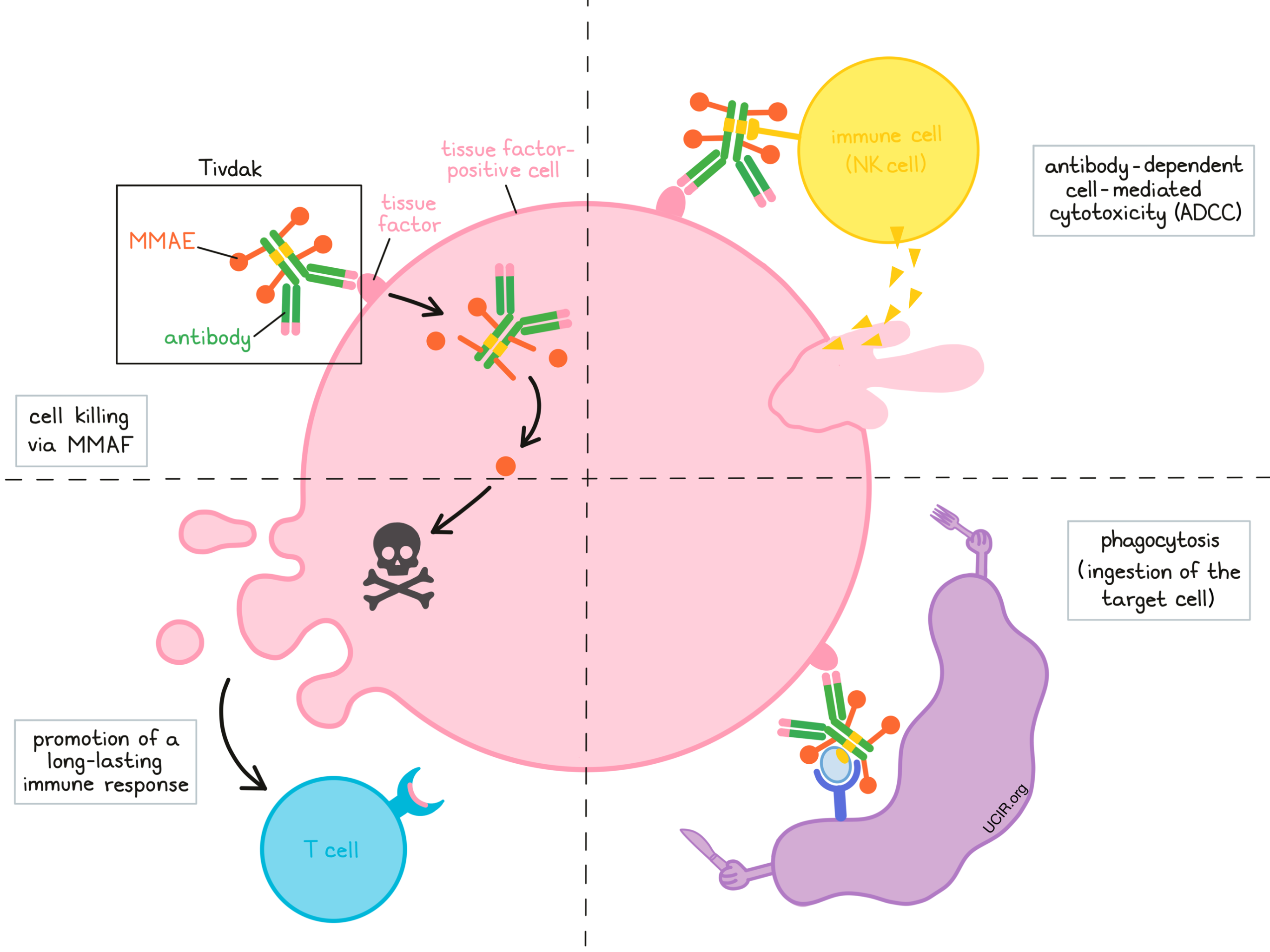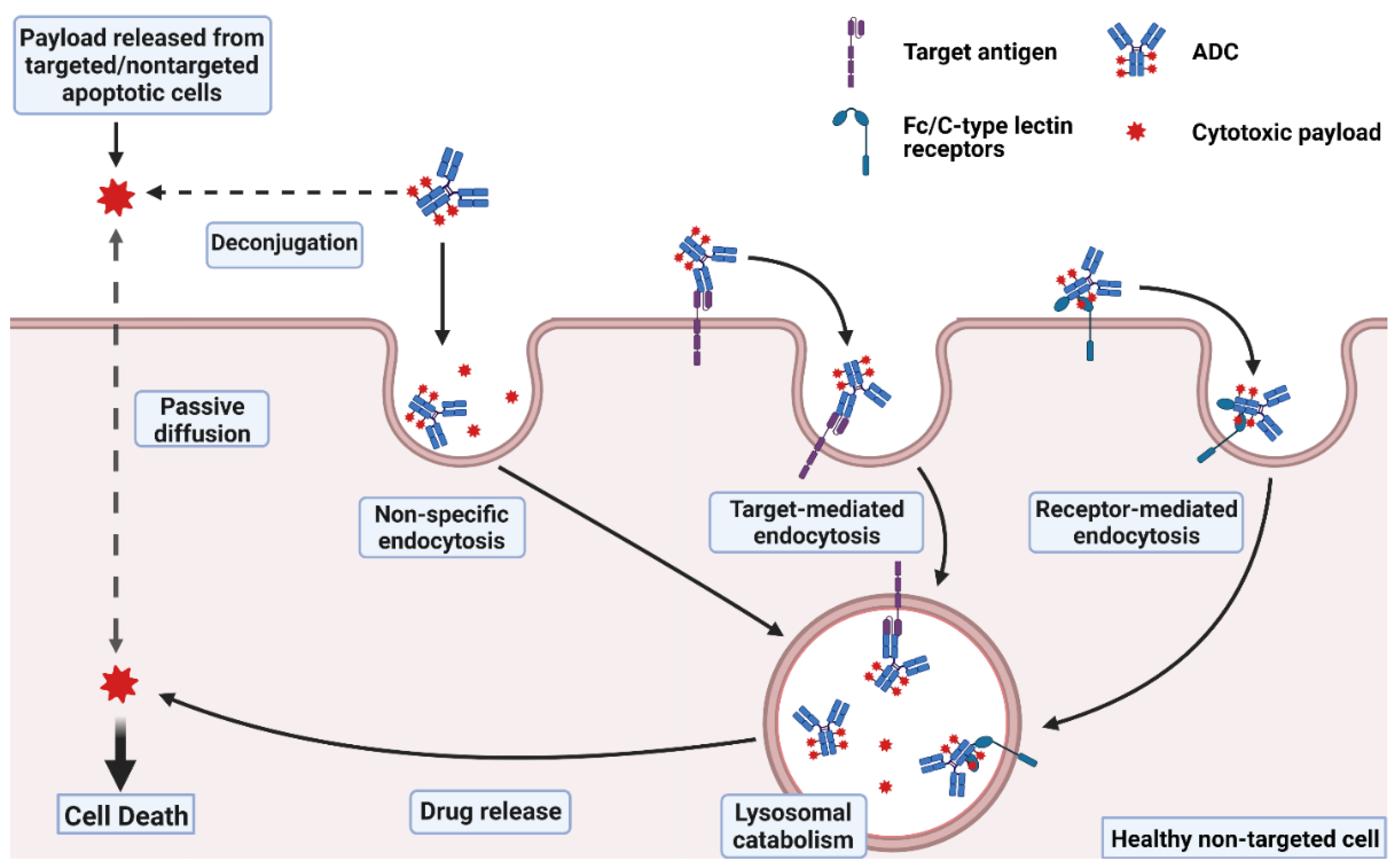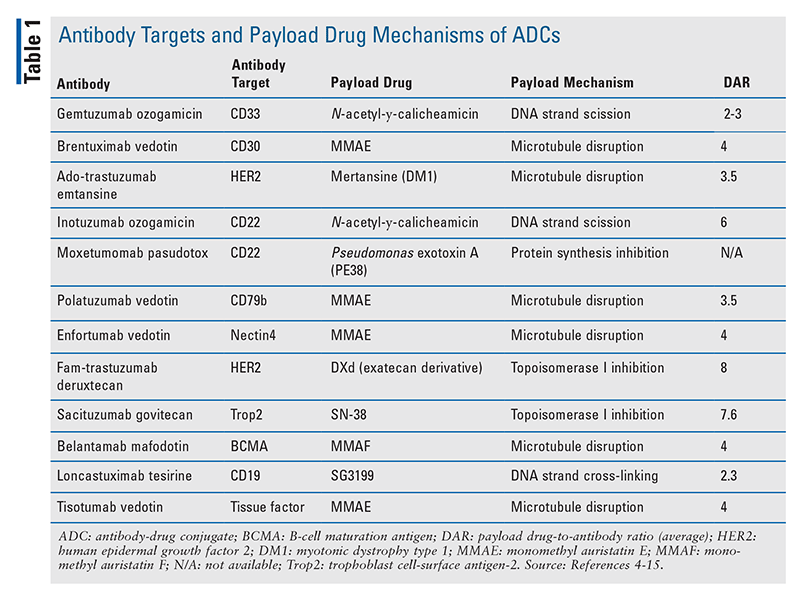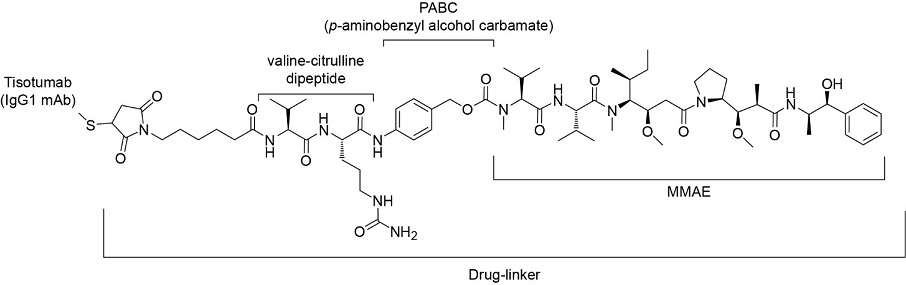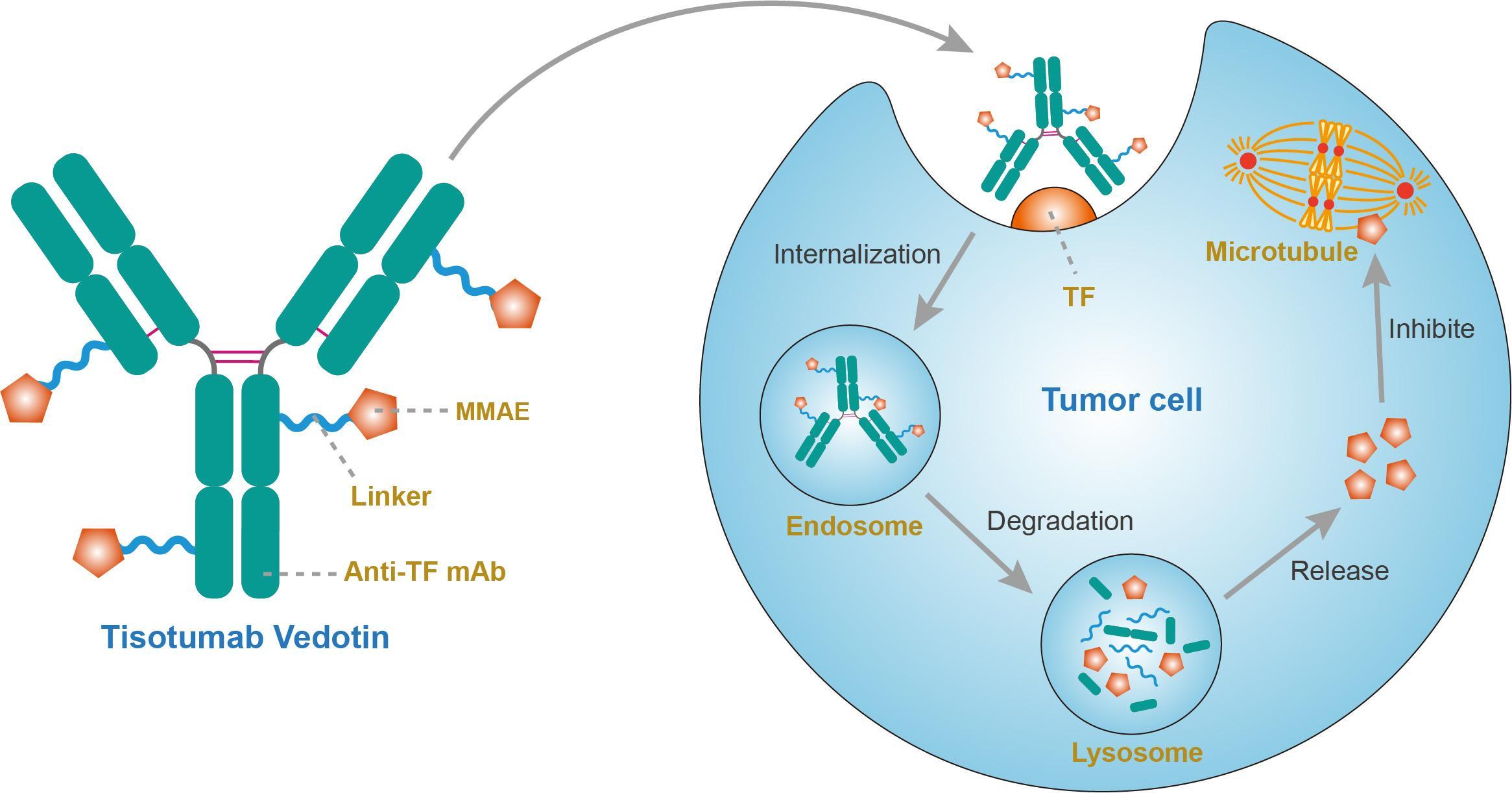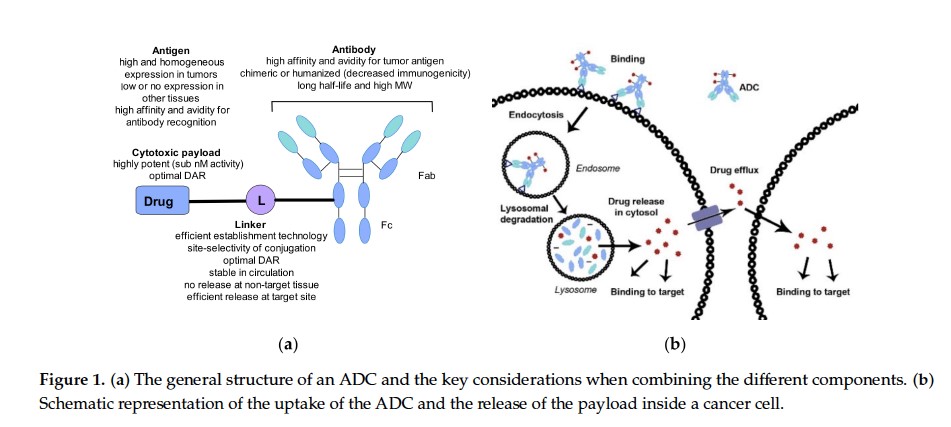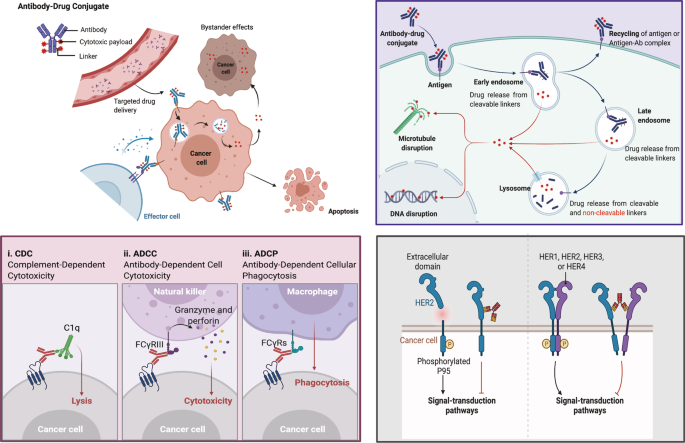
Antibody drug conjugate: the “biological missile” for targeted cancer therapy | Signal Transduction and Targeted Therapy

TIVDAK (tisotumab vedotin-tftv) compared with chemotherapy alone, met its primary endpoint of overall survival | Health | POST Online Media
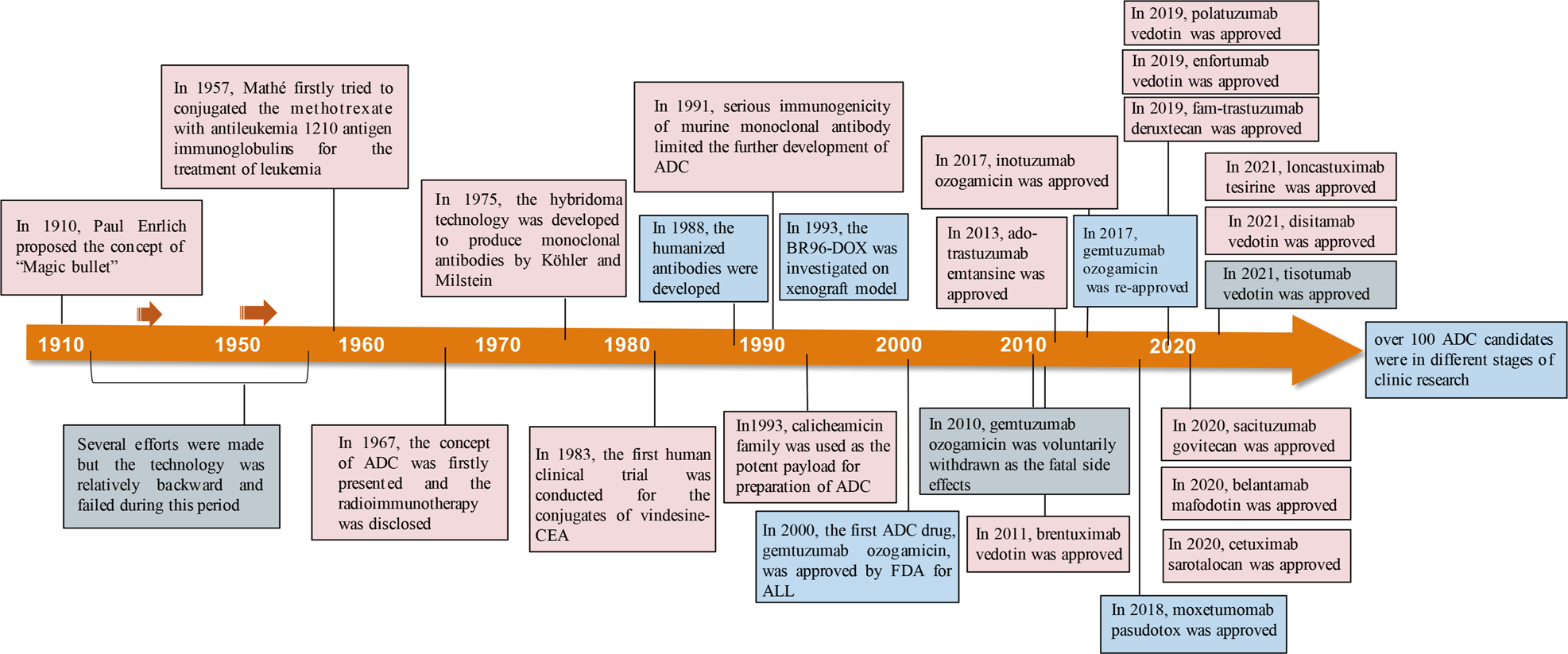
Antibody drug conjugate: the “biological missile” for targeted cancer therapy | Signal Transduction and Targeted Therapy

Genmab and Seagen Announce FDA Accelerated Approval for TIVDAK™ (tisotumab vedotin-tftv) in Previously Treated Recurrent or Metastatic Cervical Cancer | Business Wire
Genmab and Seagen Announce FDA Accelerated Approval for TIVDAK™ (tisotumab vedotin-tftv) in Previously Treated Recurrent or Me
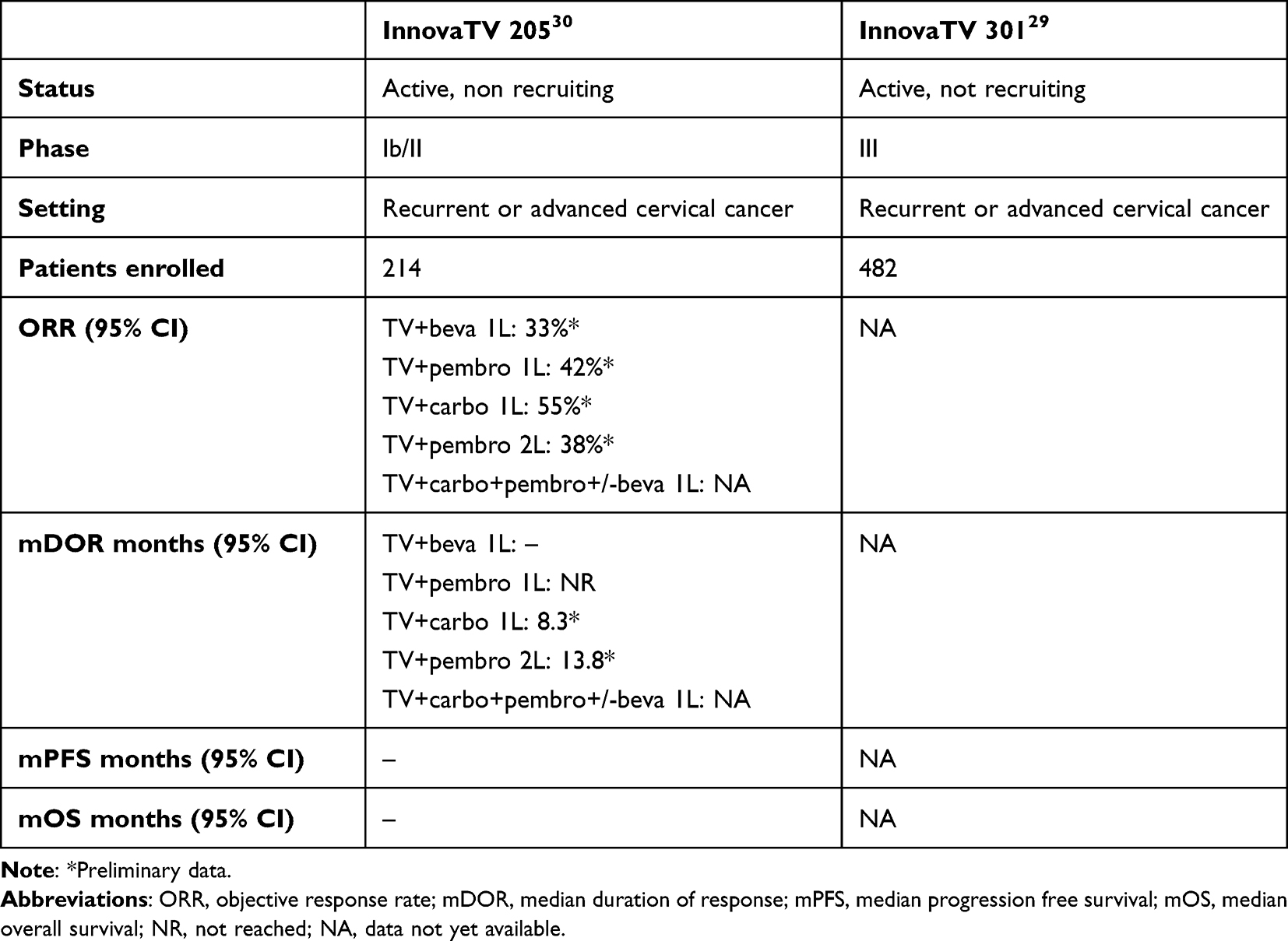
PROTOCOL AND SUMMARY OF PROTOCOL AMENDMENTS First-In-Human, Dose Escalating Safety Study of Tissue Factor Specific Antibody-Drug

Mitigation and management strategies for ocular events associated with tisotumab vedotin - ScienceDirect

PDF) Acute keratoconjunctivitis associated with tisotumab vedotin-tftv for metastatic cervical cancer