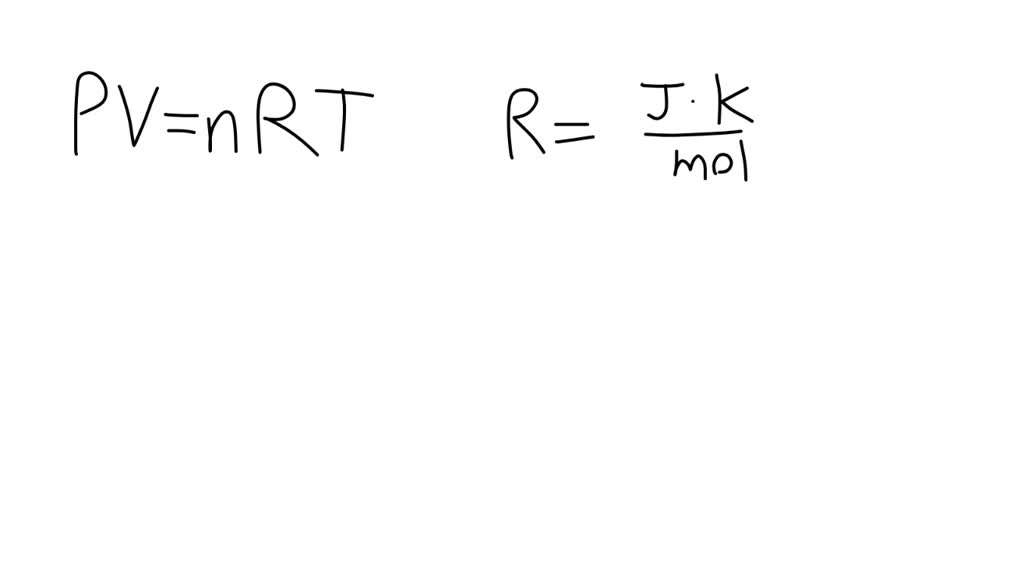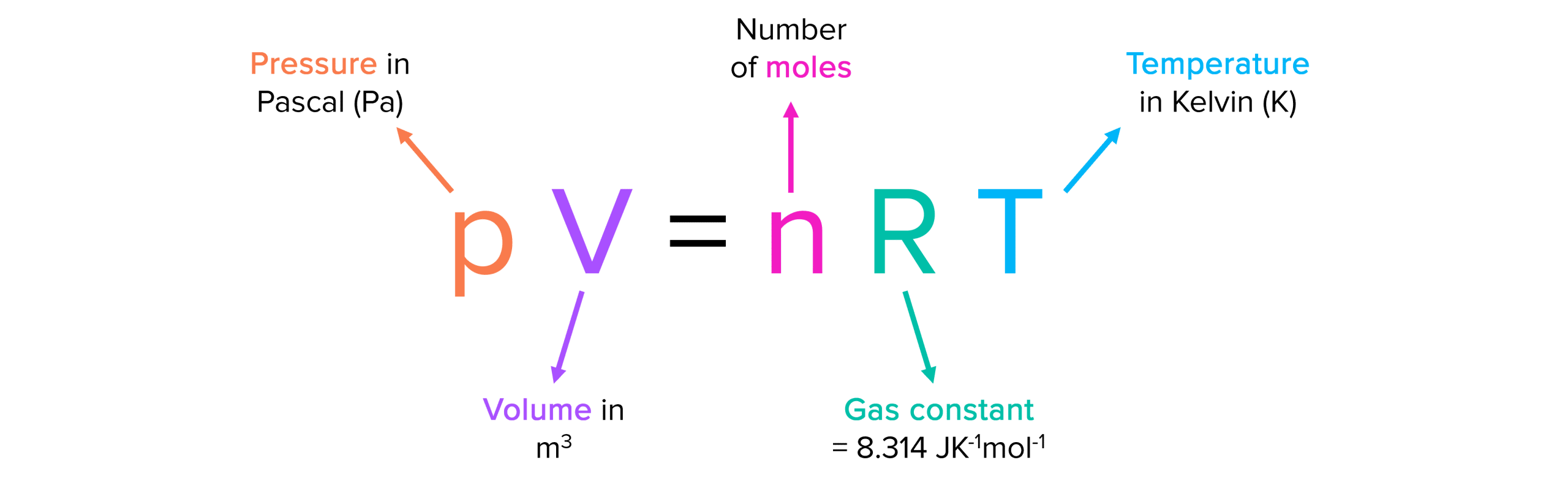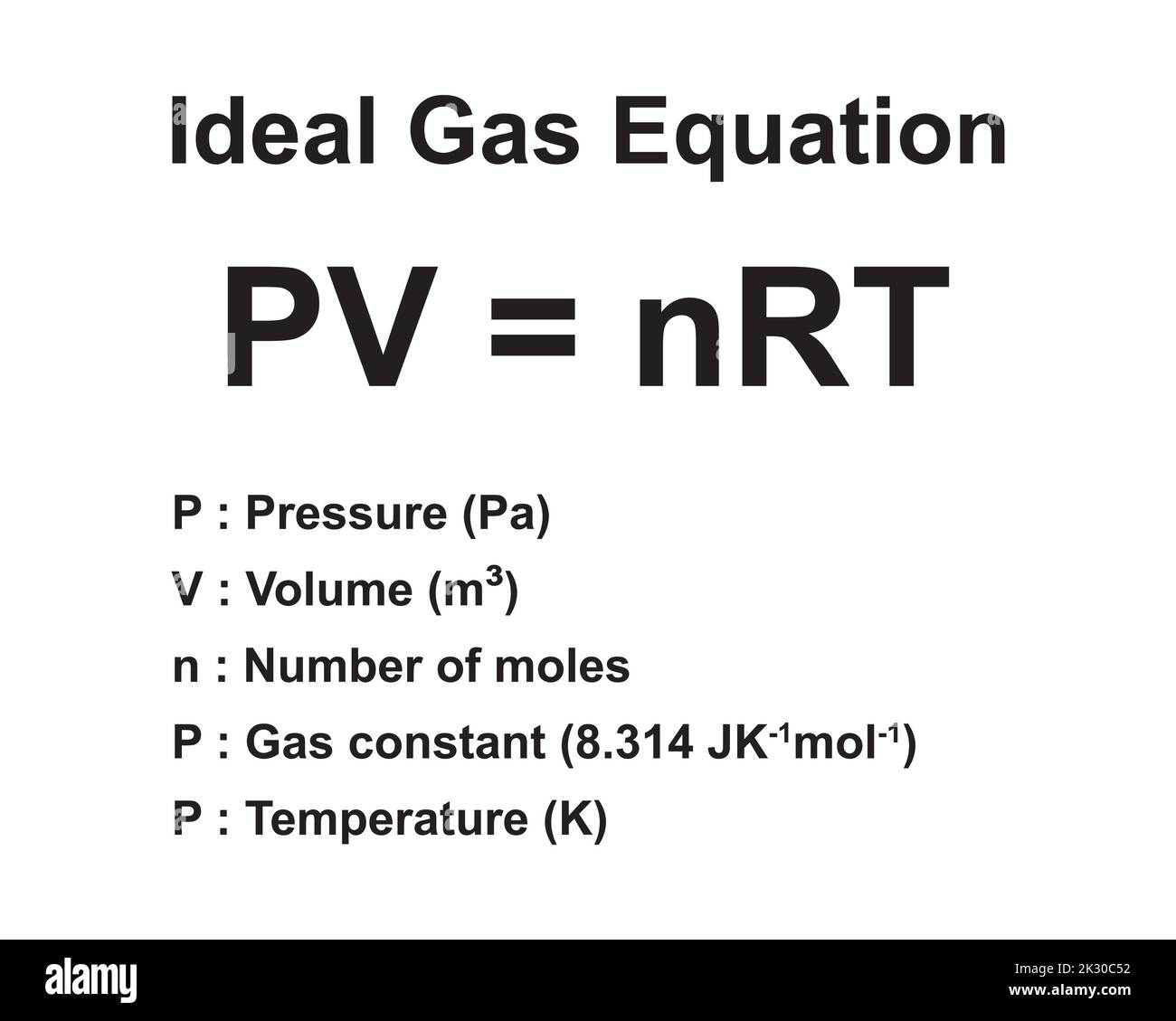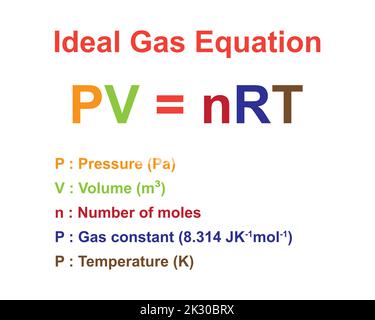
Formula ideale per la legge del gas. Pressione, volume, quantità di sostanza, costante ideale del gas e temperatura. Risorse di fisica per insegnanti e studenti Immagine e Vettoriale - Alamy

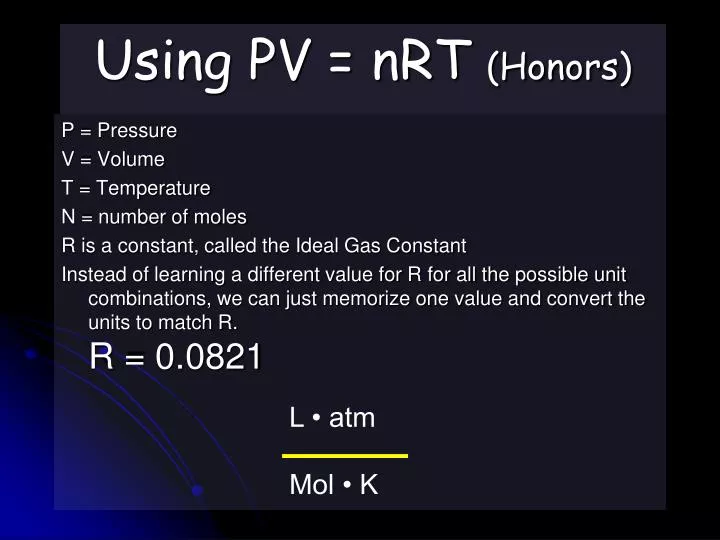
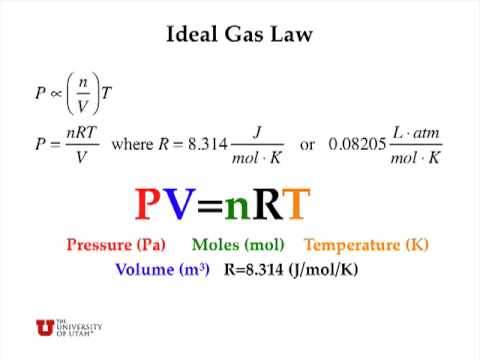
Gas Unit 3/5 The student will Learn: 1. calculation of gas problems using the Ideal Gas Law. PV = nRT. - ppt download

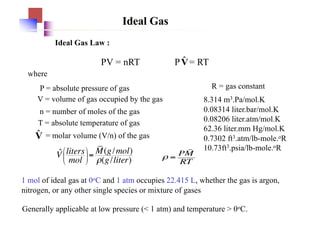
SOLVED: The ideal gas equation is PV = nRT, where P is pressure, V is volume, n is the number of moles, R is a constant, and T is temperature. You are

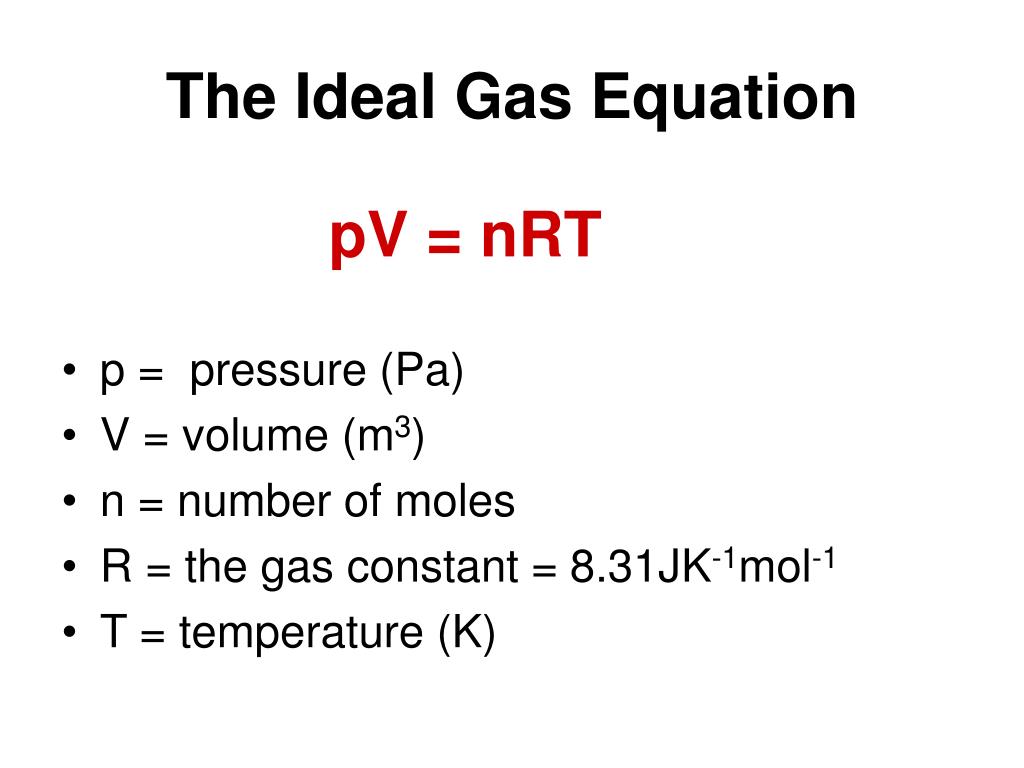
A Level Chemistry Measuring moles of GASES by measuring their volume, pressure and temperature PV = nRT. - ppt download