Applications of Sodium Nitrite in Organic Synthesis - Mukhopadhyay - 2019 - European Journal of Organic Chemistry - Wiley Online Library
How are arenediazonium salts formed when it comes to benzene reactions? What kinds of reactions can it undergo? | Socratic

Applications of Sodium Nitrite in Organic Synthesis - Mukhopadhyay - 2019 - European Journal of Organic Chemistry - Wiley Online Library

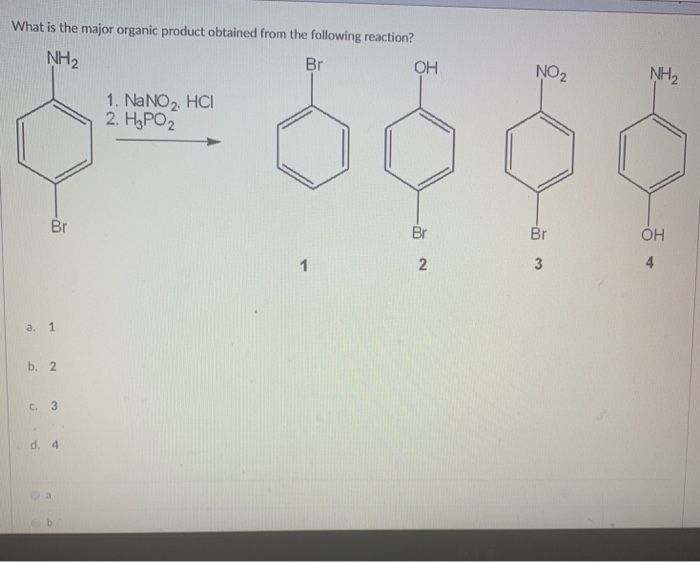
In the below given condition, intermediate “X” and reagent/condition “A” are:Aniline in the presence of NaNO2, HCl at 273−278 K forms “X” which further reacts with “A” to form phenol.

Scheme 1. Reagents and conditions: (a) (i) NaNO2, HCl, 0 °C, 30 min;... | Download Scientific Diagram
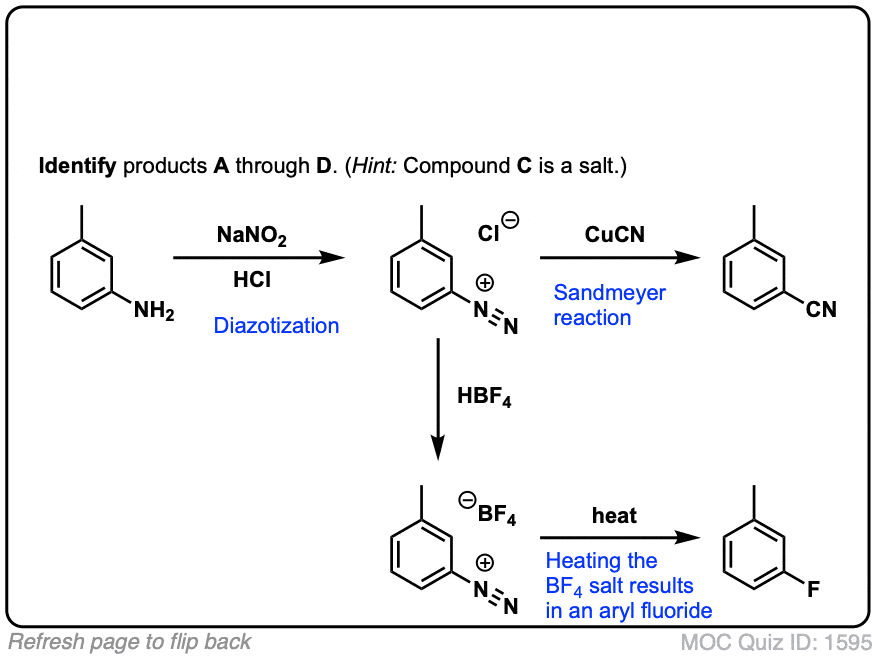
Diazotisation Reaction Substrate: Aromatic Amine Reagent: NaNO2 / HCl Reaction Temperature: 273 K Final Product: Diazonium Salt Reaction Mechanism: NO+ (Nitrosonium ion) is formed from the reaction of NaNO2 and HCl and

Molecules | Free Full-Text | Silica Sulfuric Acid/ NaNO2 as a Novel Heterogeneous System for the Nitration of Phenols under Mild Conditions

Scheme 1 Reagents and condition: (i) H2O, HCl, 80 °C; (ii) NaNO2, below... | Download Scientific Diagram

Show the detailed mechanism of the reaction of sodium sulfanilate (NH_2C_6H_4SO_3H) with HCl and NaNO_2. *a. What is the name of the organic functional group formed as an intermediate this reaction?

Which of the following is the best method for the below-mentioned conversion? a) (1)(CH3)3CCl, AlCl3, (2)I2, FeI3. b) (1)I2, FeI3, (2)(CH3)3CCl, AlCl3 c) (1)HNO3, H2SO4, (2)(CH3)3CCl, AlCl3, (3)H2, Ni, (4) NaNO2, HCl, H2O, (

organic chemistry - What is the mechanism of the reaction of a ketone with sodium nitrite/ hydrogen chloride? - Chemistry Stack Exchange
![Compound [A] is an aromatic amine which react with NaNO_{2} + HCl 273 - 278 K and form compound [B]. Compound [B] react with HBF_{4} and the obtain product on further heating, Compound [A] is an aromatic amine which react with NaNO_{2} + HCl 273 - 278 K and form compound [B]. Compound [B] react with HBF_{4} and the obtain product on further heating,](https://search-static.byjusweb.com/question-images/toppr_ext/questions/774137_738479_ans_d47545d3f5ad465d9fd600a977e7df56.png)