Medical Device Single Audit Program (MDSAP) - Audit Processes | Freyr - Global Regulatory Solutions and Services Company

IMQ is the only Italian body recognised as an Authorised Auditing Organisation for MDSAP (Medical Device Single Audit Program). - IMQ

Know About Common Non-conformities to MDSAP Audit (Get Tips for Successful MDSAP Audit) | Operon Strategist

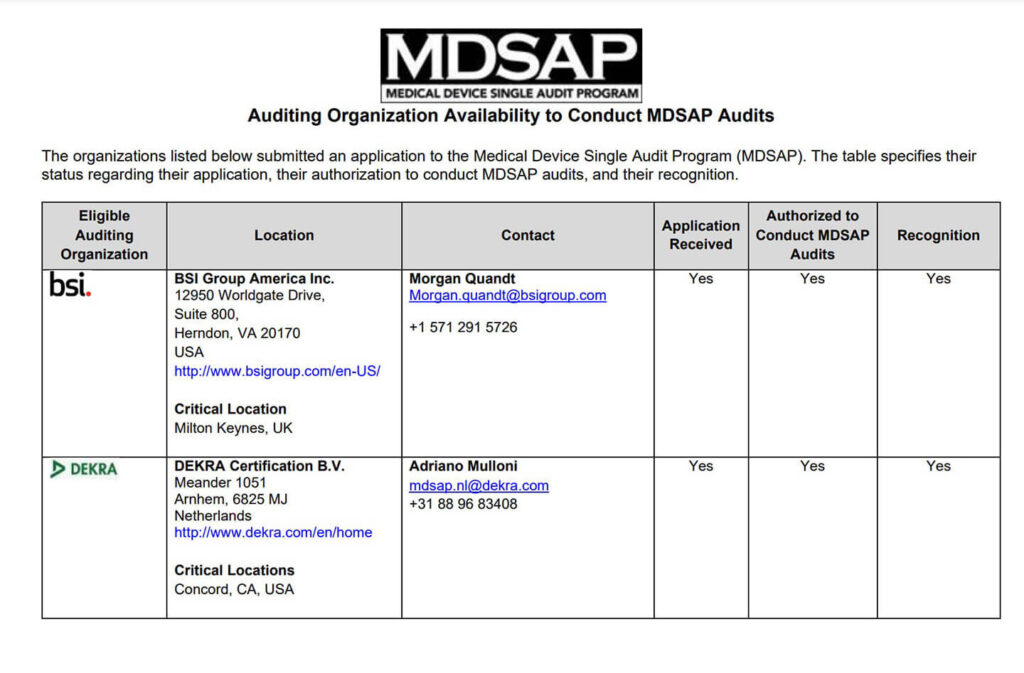
Guidance Document: Requirements in the Recognition Process for Medical Device Single Audit Program (MDSAP) Auditing Organizations - Canada.ca

The Importance of the Medical Device Single Audit Program (MDSAP) and EU MDR Lead Auditor Certifications - EAS Consulting Group

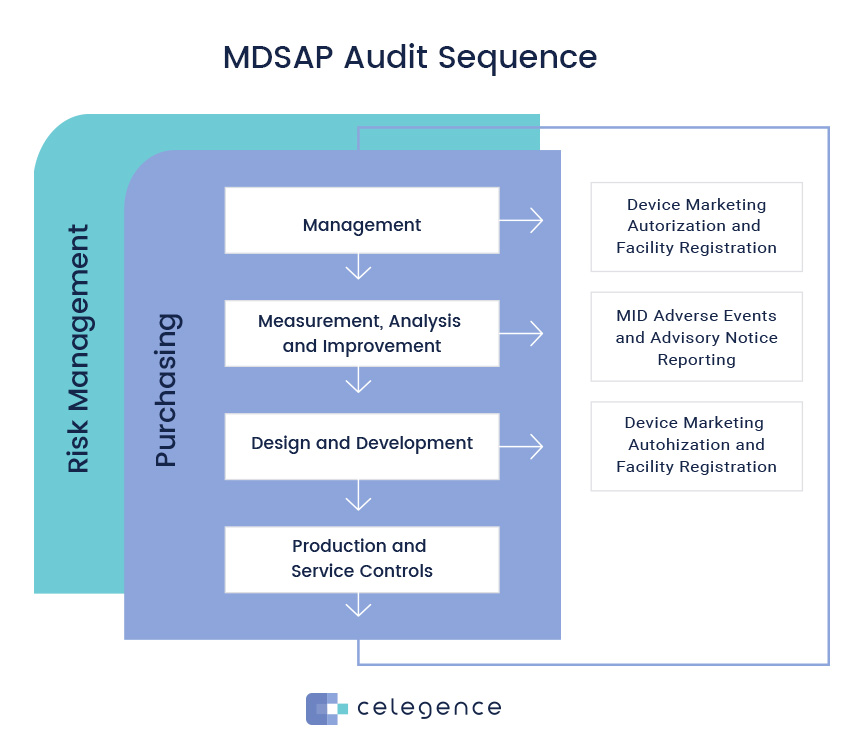
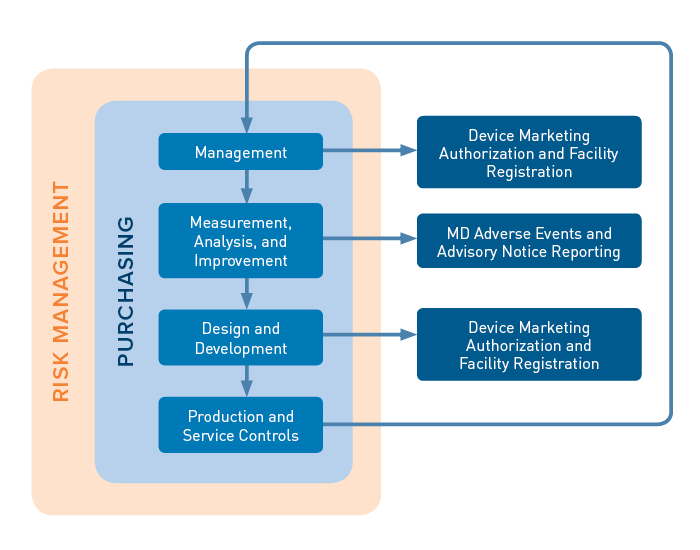
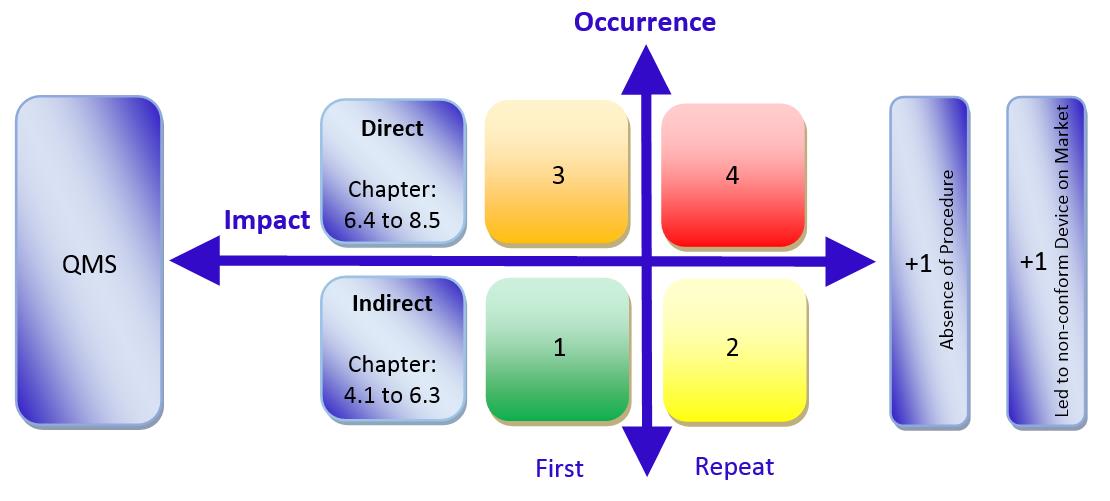
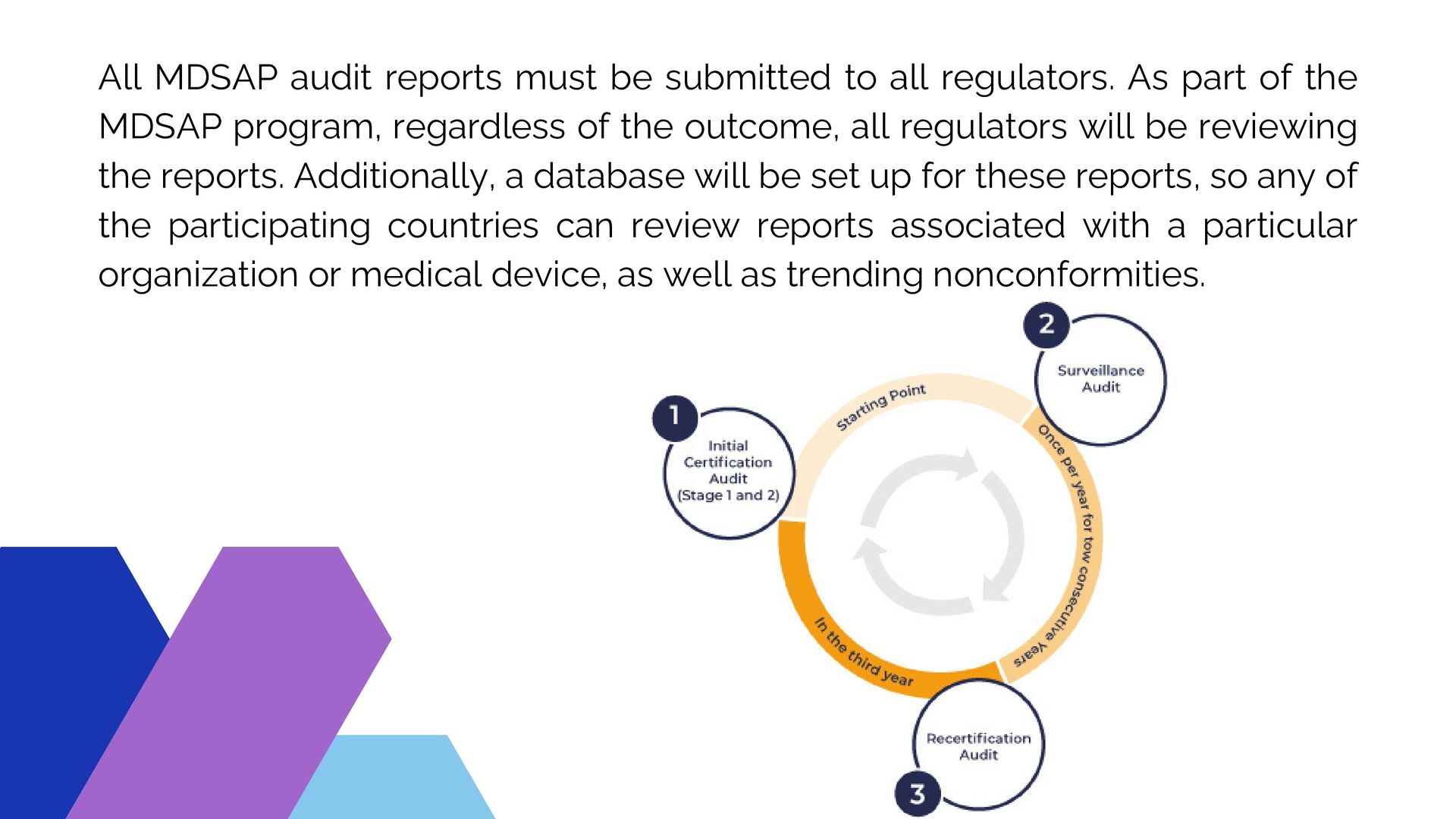
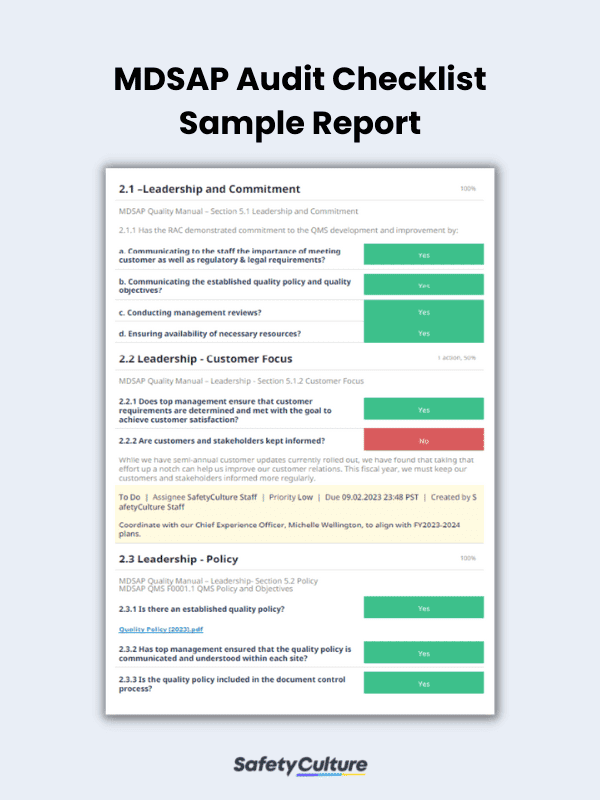


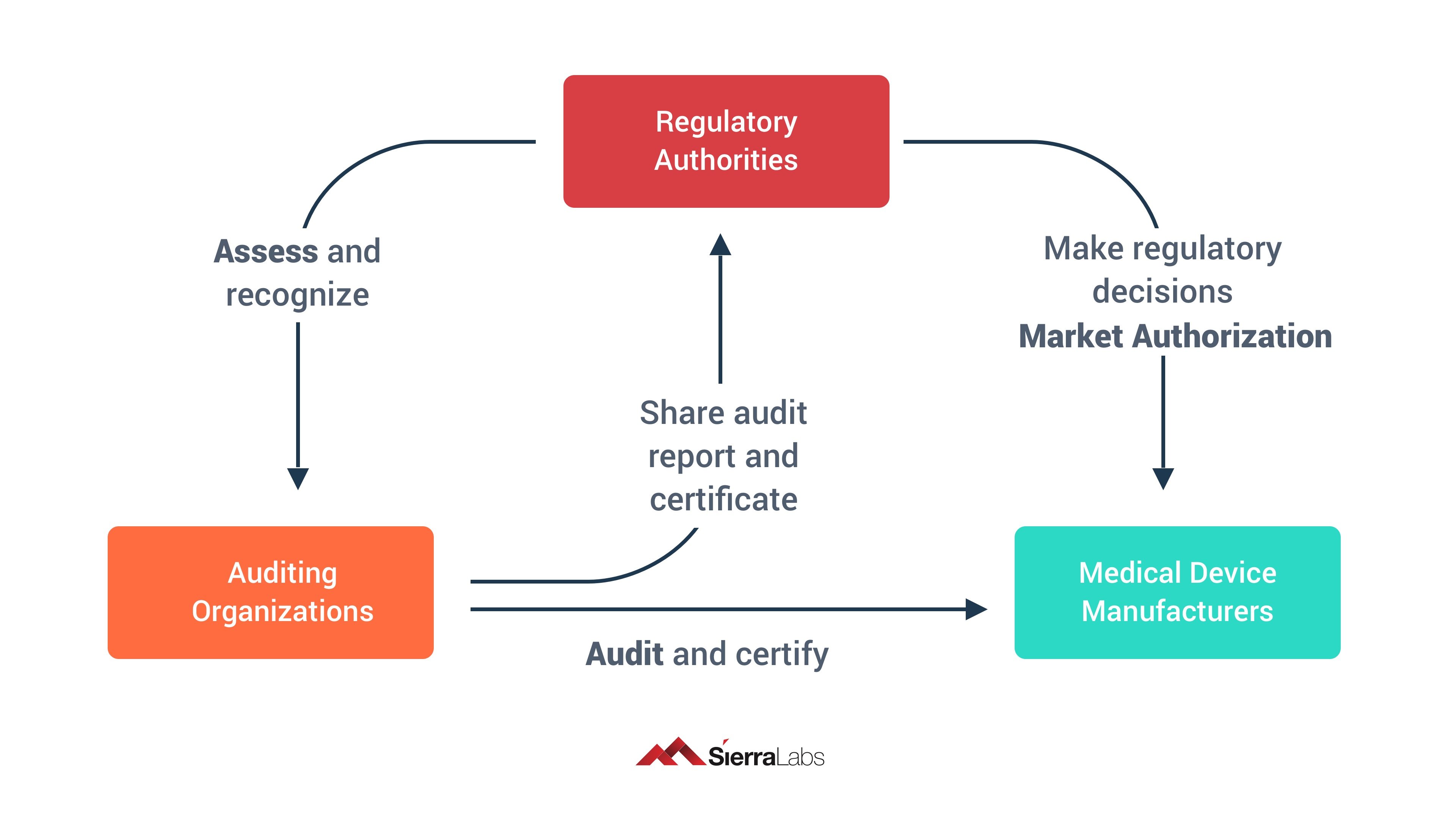

/tuv-rheinland_mdsap-visual-en-update.png)