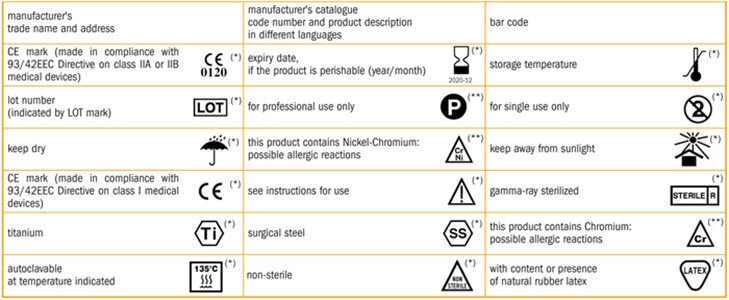
IFU (Instructions for Use), includes printed materials such as booklets and inserts required for products, medical devices, and pharmaceuticals to fulfill government requirements.
IFU (Instructions for Use), includes printed materials such as booklets and inserts required for products, medical devices, and pharmaceuticals to fulfill government requirements.

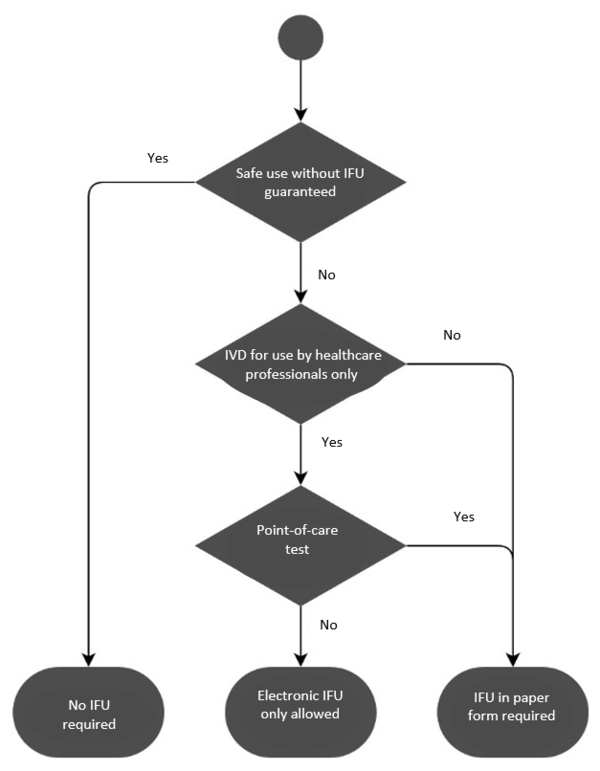
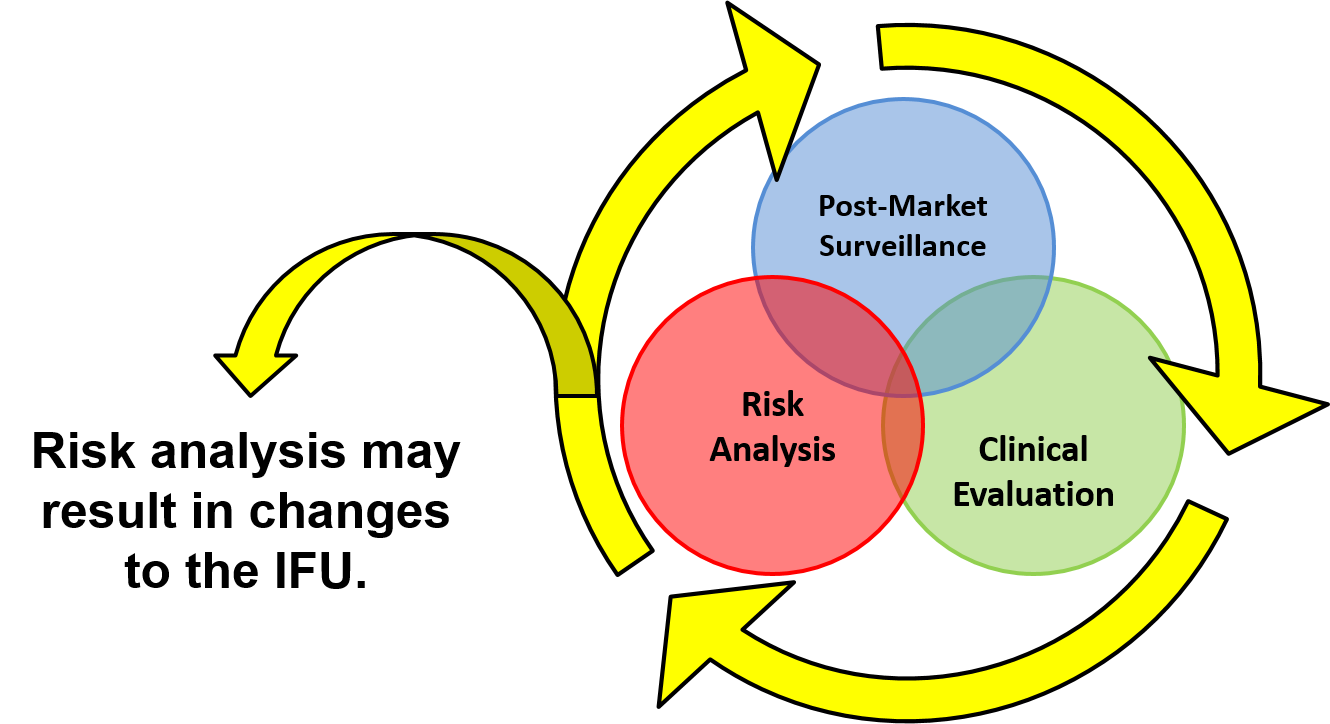
Implementing a compliant solution for the electronic distribution of Instructions for Use - Medical Device Network