Compendium of Hydrogen Energy: Hydrogen Storage, Distribution and Infrastructure (Woodhead Publishing Series in Energy Book 2) (English Edition) eBook : Gupta, Ram, Basile, Angelo, Veziroglu, T. Nejat: Amazon.it: Kindle Store

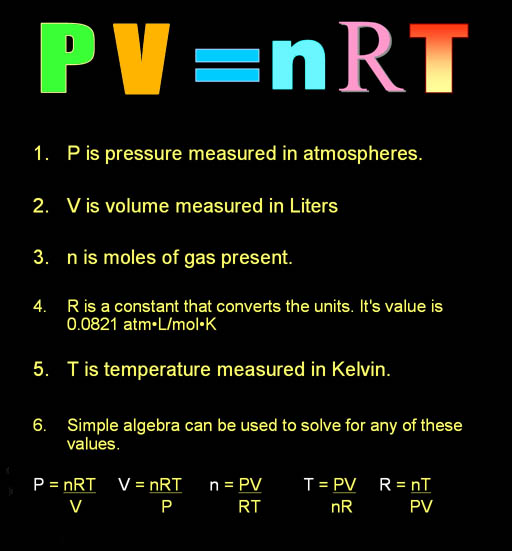
Specific heat of one mole of hydrogen at constant pressure and at constant volume are 450 JK^-1 and 300 JK^-1 respectively. Then what is the density of the gas at S.T.P ? ( Patm = 1.013 × 10^5Nm^-2 )
Hydrogen | Free Full-Text | Aspects of Hydrogen and Biomethane Introduction in Natural Gas Infrastructure and Equipment

The volume of 1kg of hydrogen gas at N.T.P. is `11.2m^(3)`. Specific heat of hydrogen at constant - YouTube
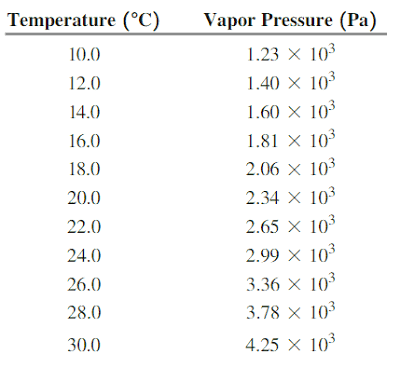
A volume of hydrogen gas is collected over water in an investigation. If the atmospheric pressure is 102.1 kPa and the pressure of the hydrogen gas is 100.1 kPa, what temperature is

An exact pressure solution for hydrogen constant volume heating from a... | Download Scientific Diagram

An exact pressure solution for hydrogen constant volume heating from a... | Download Scientific Diagram

PureCareRx | Hydrogen Peroxide 6 Percent | 20 Volume Developer | Hydrogen Peroxide Gallon | 6% Hydrogen Peroxide | 20 Volume Hydrogen Peroxide | 32oz Bottle: Amazon.com: Industrial & Scientific

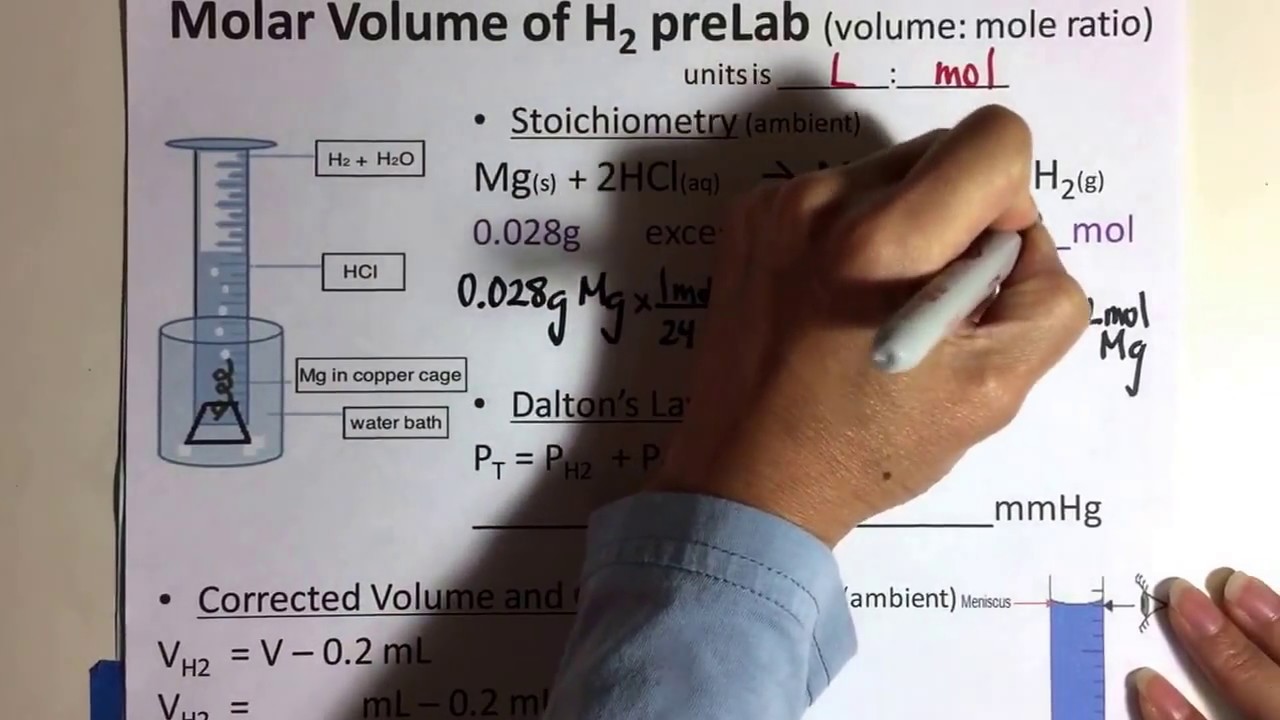
SOLVED: Gas/bubbles came from the magnesium. Then magnesium completely dissolved. Volume of hydrogen gas collected (mL) 13.00 Volume of hydrogen gas collected (L) 0.01300 Pressure of hydrogen gas (mmHg) 754.2 Pressure of

A graph of volume of hydrogen released vs time for the reaction between zinc and dil. HCl is given in the figure. On the basis of this mark the correct option.a Average