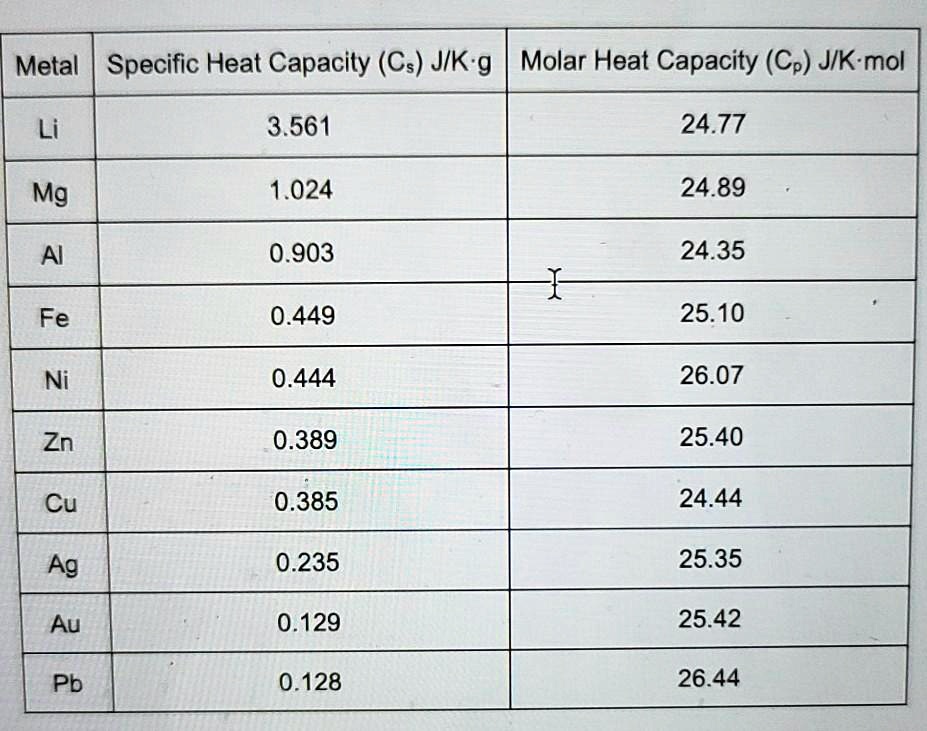
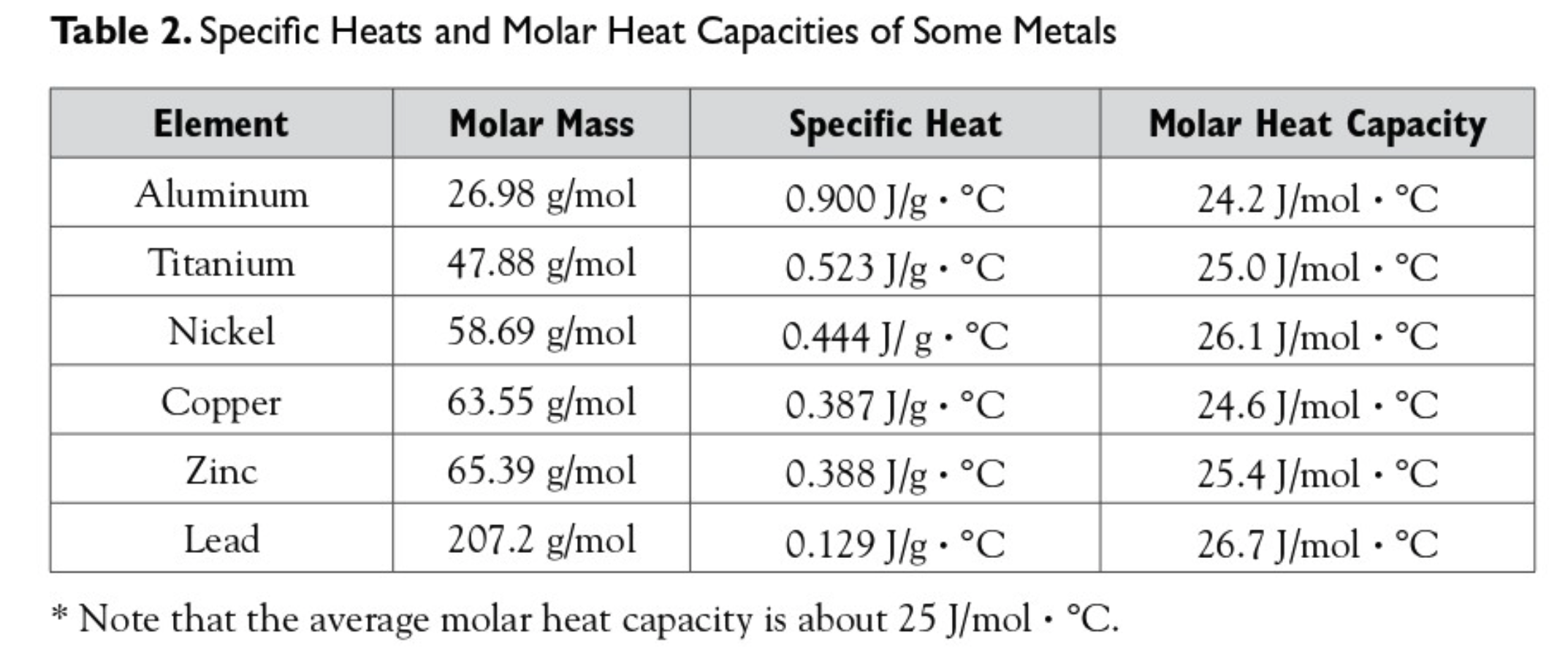
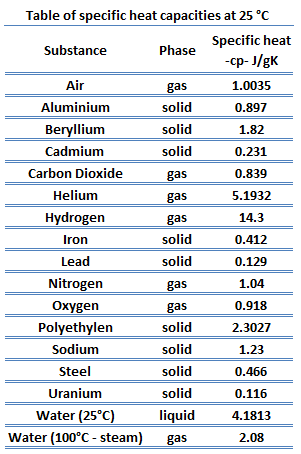
Observe the following diagram and answer the questions given below: Which element has maximum specific heat capacity? Justify. - Science and Technology 1 | Shaalaa.com
![PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b0d76592f078aeeb6e66f500b45a1f101c6fe150/7-Table4-1.png)
PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar

PDF) A new correlation for the specific heat of metals, metal oxides and metal fluorides as a function of temperature

Metals | Free Full-Text | Thermodynamic Properties and Equation of State for Solid and Liquid Aluminum
![PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b0d76592f078aeeb6e66f500b45a1f101c6fe150/3-Figure1-1.png)
PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar

SOLVED: Table 1. Specific heat capacities and densities of common metals Metal Specific Heat Capacity (J/g°C) Density (g/cm³) Iron 0.45 7.87 Aluminum 0.91 2.70 Lead 0.13 11.36 Copper 0.96 8.96 Zinc 7.13 Tin 0.21 7.28 Gold 19.32

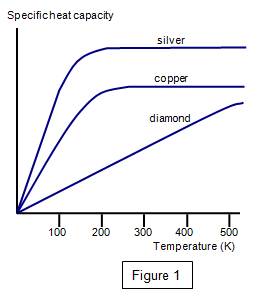
Specific heat capacity of metals at low temperatures.(1 cal = 4.18 J)... | Download Scientific Diagram
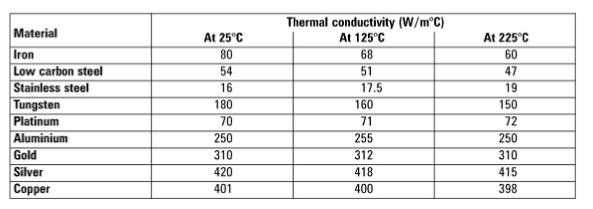
Which metal achieves the highest temperature when adding energy? Aluminum, Copper, or Silver? | CIDER
How does heat capacity of copper (or aluminum) change with temperature, when the temperature is in the region of 0°C to 80°C and why? - Quora
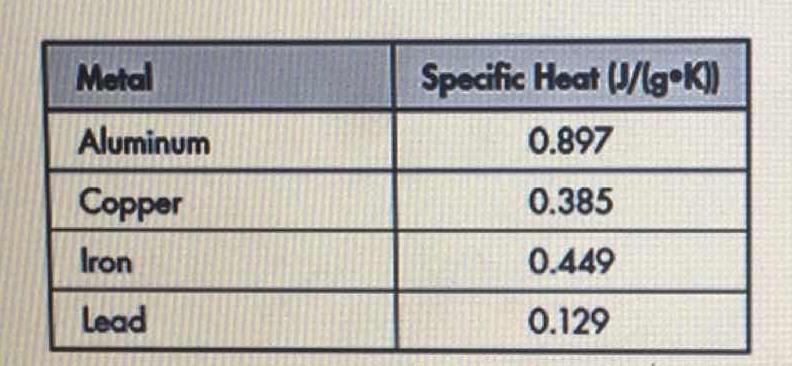
The table below shows the specific heats of several metals. The temperature of a 15-g sample of an unknown metal increases from 20.0 C to 30.0 C when it absorbs 67.5 J