
10 .Csir net December 2017 organic chemistry solution Iodo lactonization,DBU reagent in hindi - YouTube

Three‐Component Perfluoroalkylvinylation of Alkenes Enabled by Dual DBU/Fe Catalysis** - Tang - 2023 - Chemistry – A European Journal - Wiley Online Library
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity. | Semantic Scholar The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7f11e50e3c561e40fc500f0bf3918481d2ad6938/1-Figure1-1.png)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity. | Semantic Scholar
![Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/da071cf8-eee0-4e2f-8e22-5b6f6a0f91e7/adsc201601279-fig-5004-m.jpg)
Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library
![The Reaction of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) with Carbon Dioxide | The Journal of Organic Chemistry The Reaction of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) with Carbon Dioxide | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/jo0503759/asset/images/jo0503759.social.jpeg_v03)
The Reaction of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) with Carbon Dioxide | The Journal of Organic Chemistry

Frontiers | CO2 Absorption by DBU-Based Protic Ionic Liquids: Basicity of Anion Dictates the Absorption Capacity and Mechanism
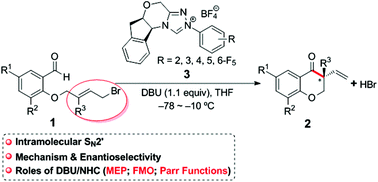
Theoretical study on the mechanism and enantioselectivity of NHC-catalyzed intramolecular SN2′ nucleophilic substitution: what are the roles of NHC and DBU? - Organic Chemistry Frontiers (RSC Publishing)
![Figure 1 from 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) and microwave-accelerated green chemistry in methylation of phenols, indoles, and benzimidazoles with dimethyl carbonate. | Semantic Scholar Figure 1 from 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) and microwave-accelerated green chemistry in methylation of phenols, indoles, and benzimidazoles with dimethyl carbonate. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0d4133dd6314ab3a6af35d9a5101b828f28a7059/2-Figure1-1.png)
Figure 1 from 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) and microwave-accelerated green chemistry in methylation of phenols, indoles, and benzimidazoles with dimethyl carbonate. | Semantic Scholar
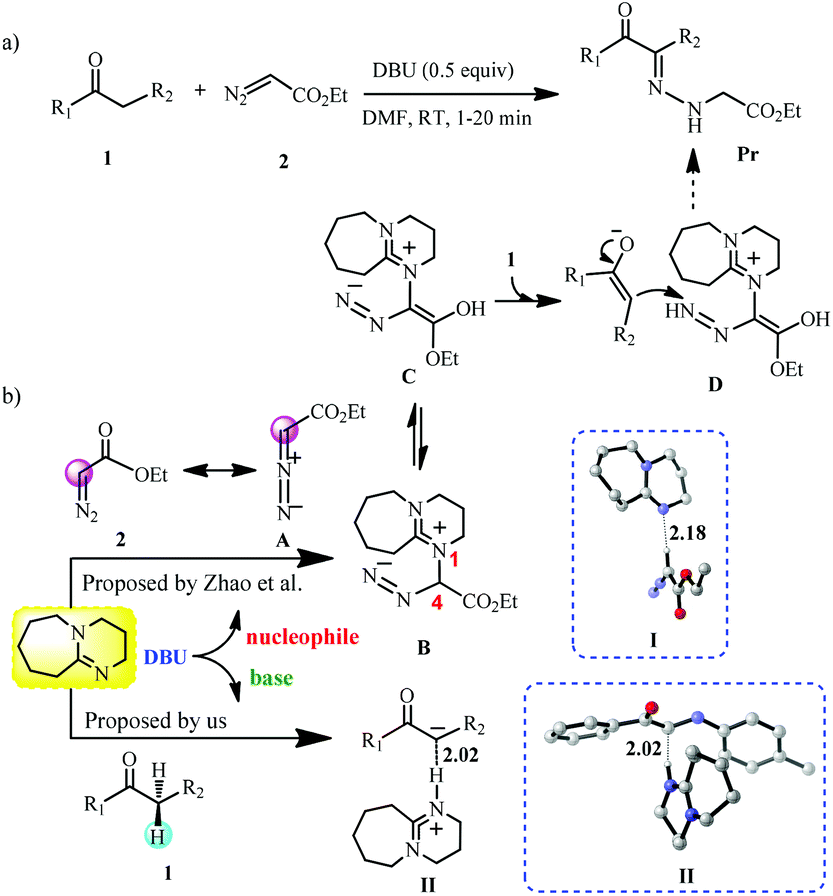
Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA ... - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C9QO00602H
![1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science](https://www.eurekaselect.com/images/graphical-abstract/coc/19/9/0005D.gif)
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science
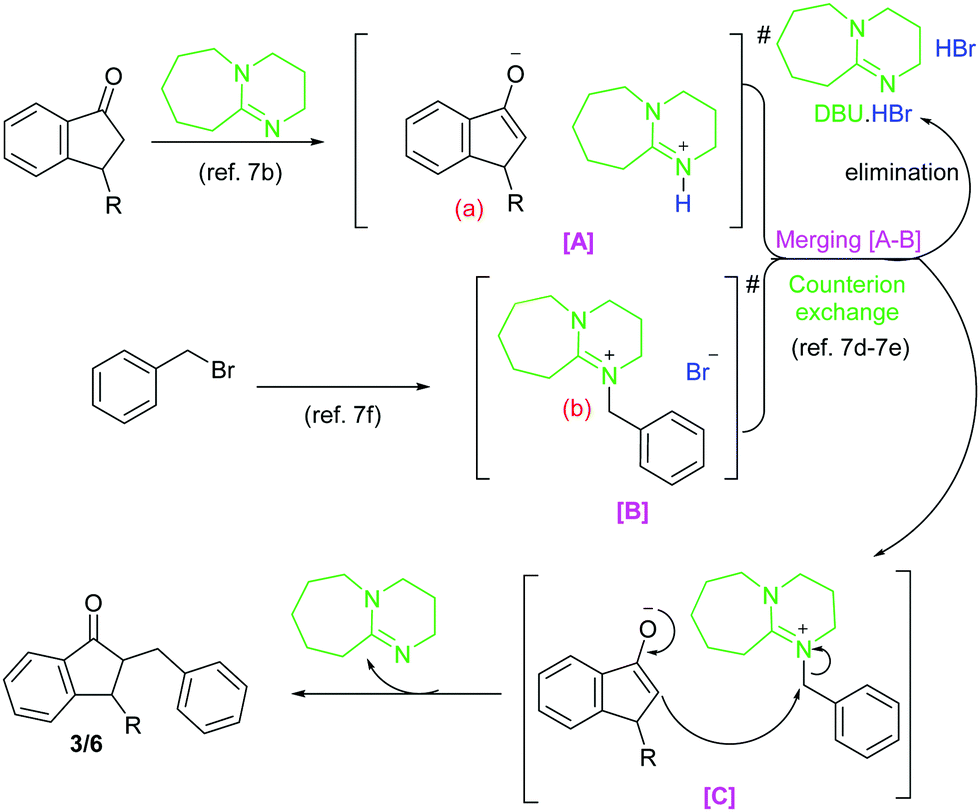
An efficient merging of DBU/enolate and DBU/benzyl bromide organocycles for the synthesis of alpha benzylated 1-indanone derivatives - New Journal of Chemistry (RSC Publishing) DOI:10.1039/D2NJ00783E




![1,8-Diazabicyclo[5.4.0]undec-7-ene | C9H16N2 - PubChem 1,8-Diazabicyclo[5.4.0]undec-7-ene | C9H16N2 - PubChem](https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=81184&t=l)
![1,8-Diazabicyclo[5.4.0]undec-7-ene, 98 %, Thermo Scientific Chemicals | Fisher Scientific 1,8-Diazabicyclo[5.4.0]undec-7-ene, 98 %, Thermo Scientific Chemicals | Fisher Scientific](https://assets.fishersci.com/TFS-Assets/CCG/Chemical-Structures/chemical-structure-cas-6674-22-2.jpg-650.jpg)

![Synthesis of Azobenzenes Using N-Chlorosuccinimide and 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) Synthesis of Azobenzenes Using N-Chlorosuccinimide and 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)](https://www.organic-chemistry.org/abstracts/lit6/017o.gif)