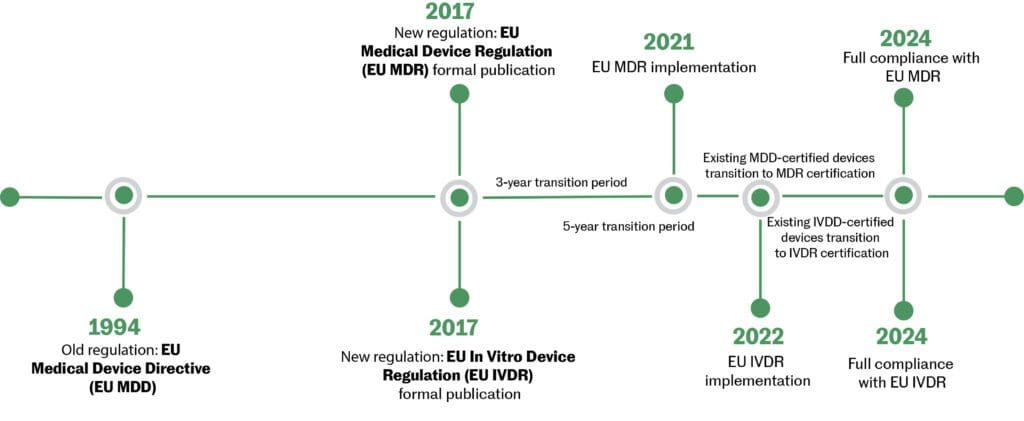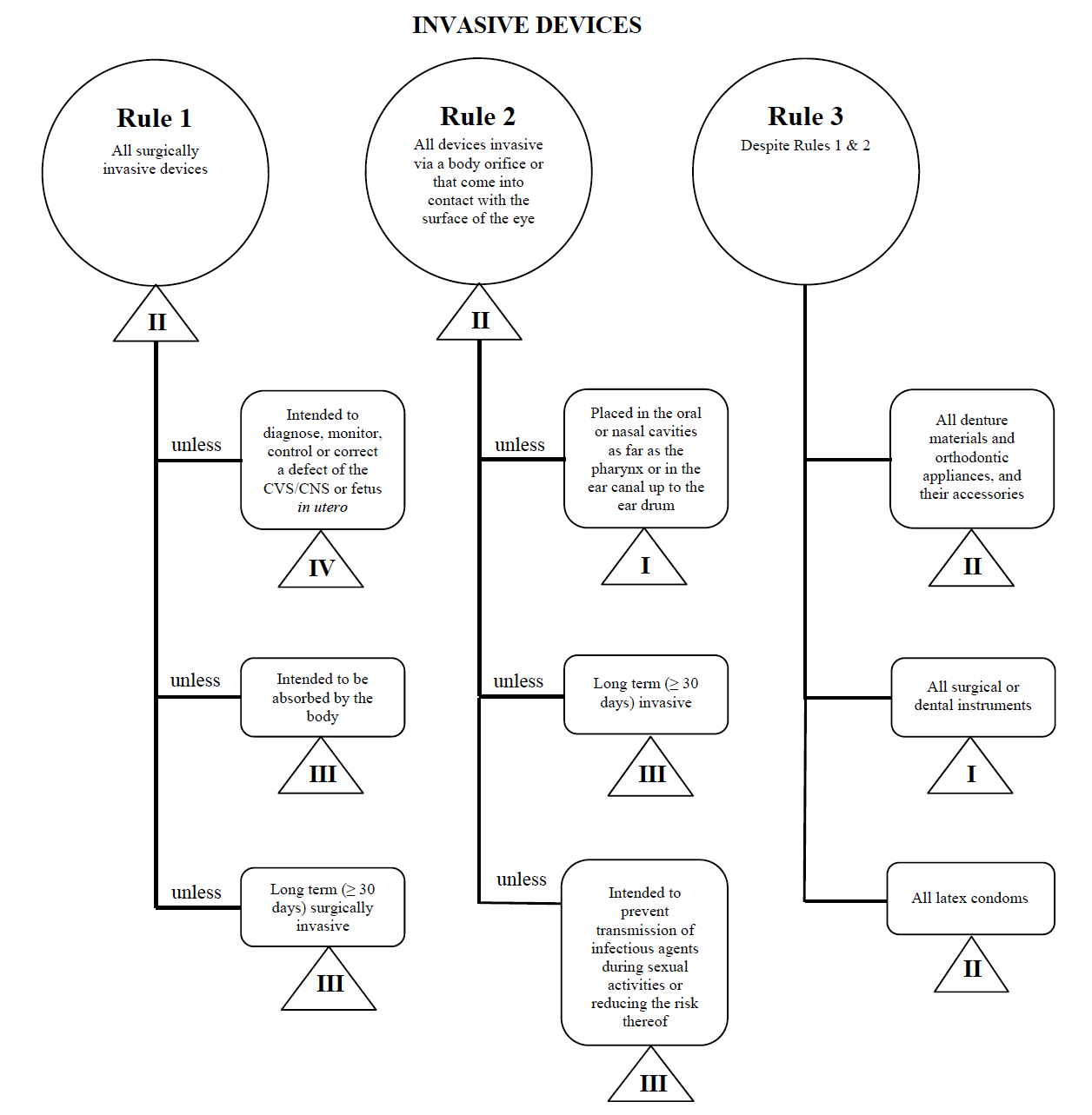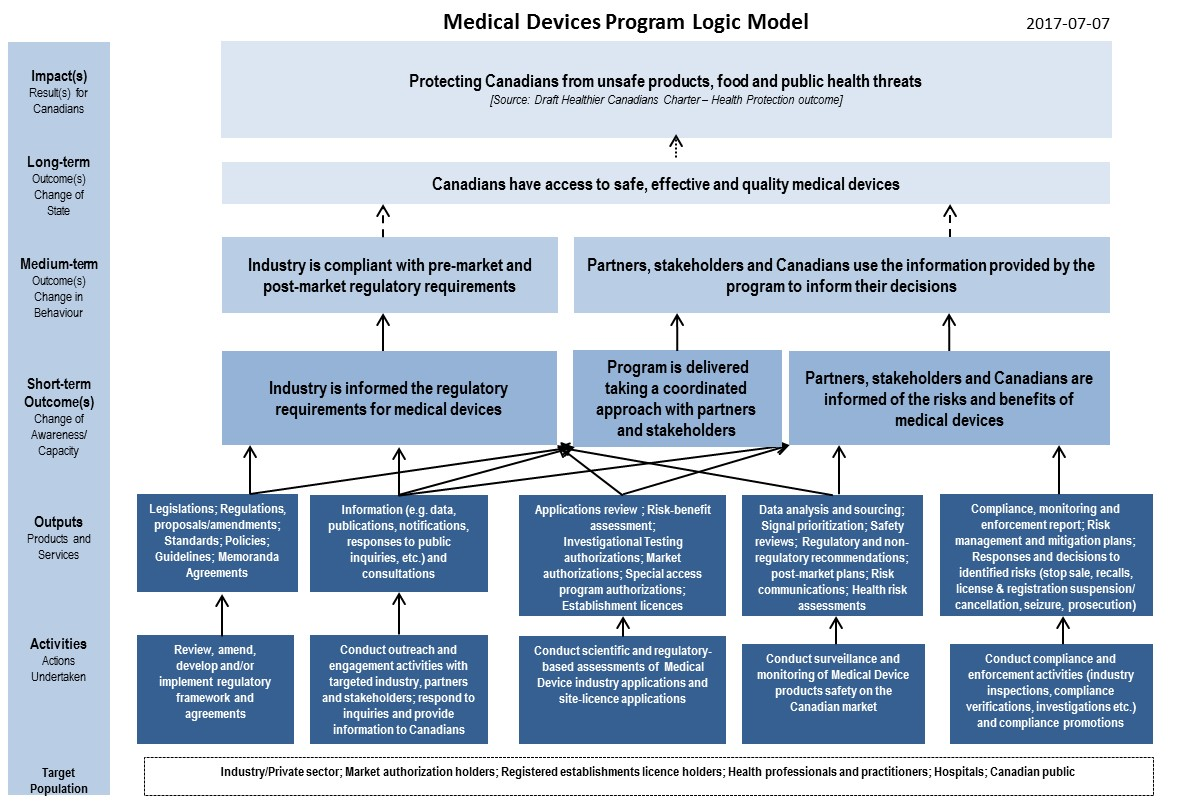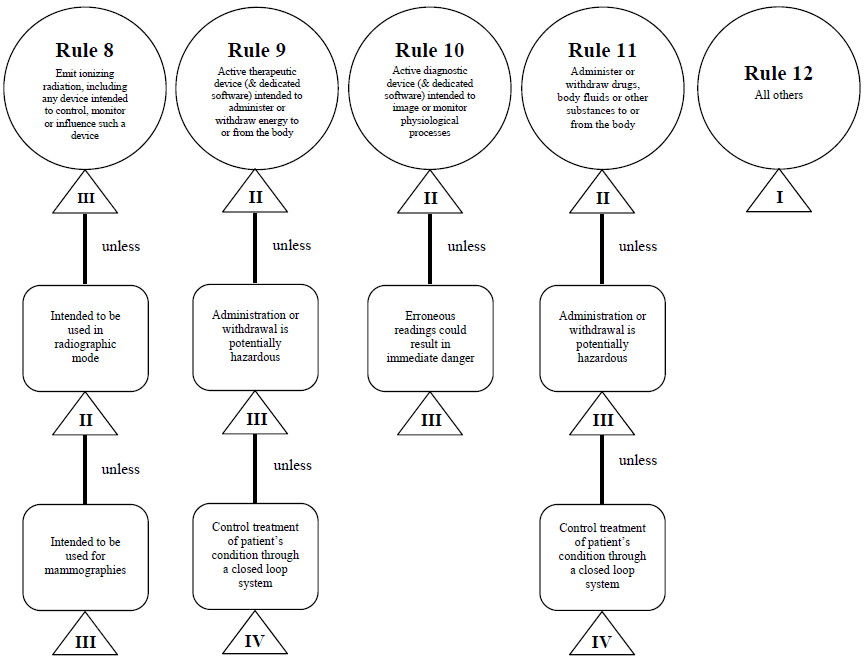
Guidance Document - Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs) - Canada.ca

Overview of Health Canada regulations for medical devices and examples... | Download Scientific Diagram

Seminar: Medical Device Regulatory changes for Canada, USA, UK and EU | September 22, 2023 - Centech

Current state of Health Canada regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy